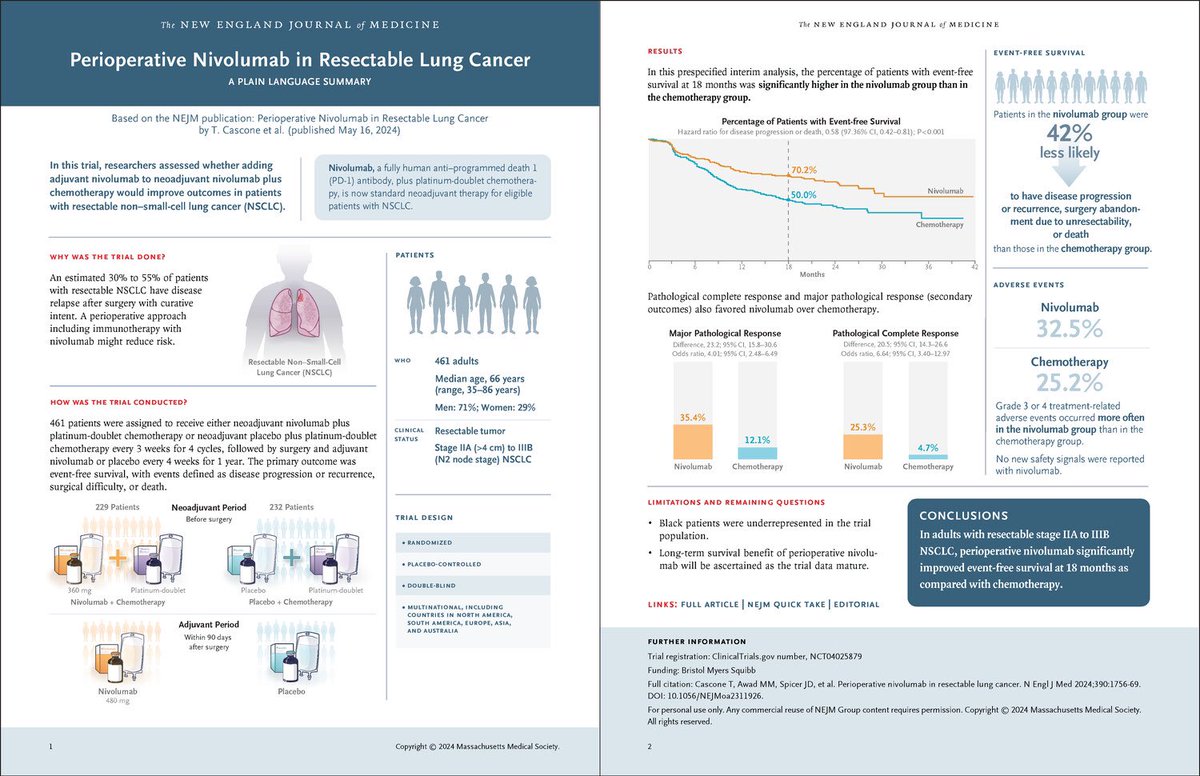
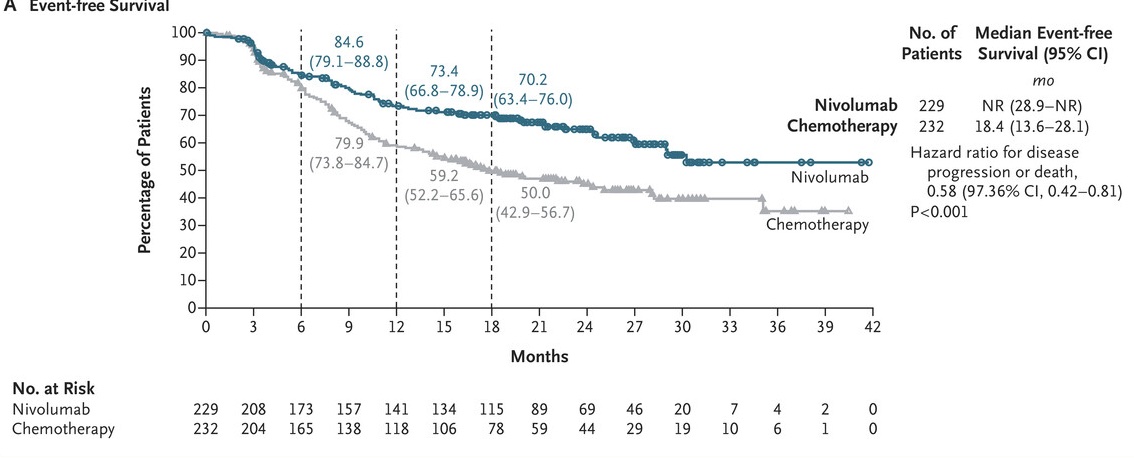
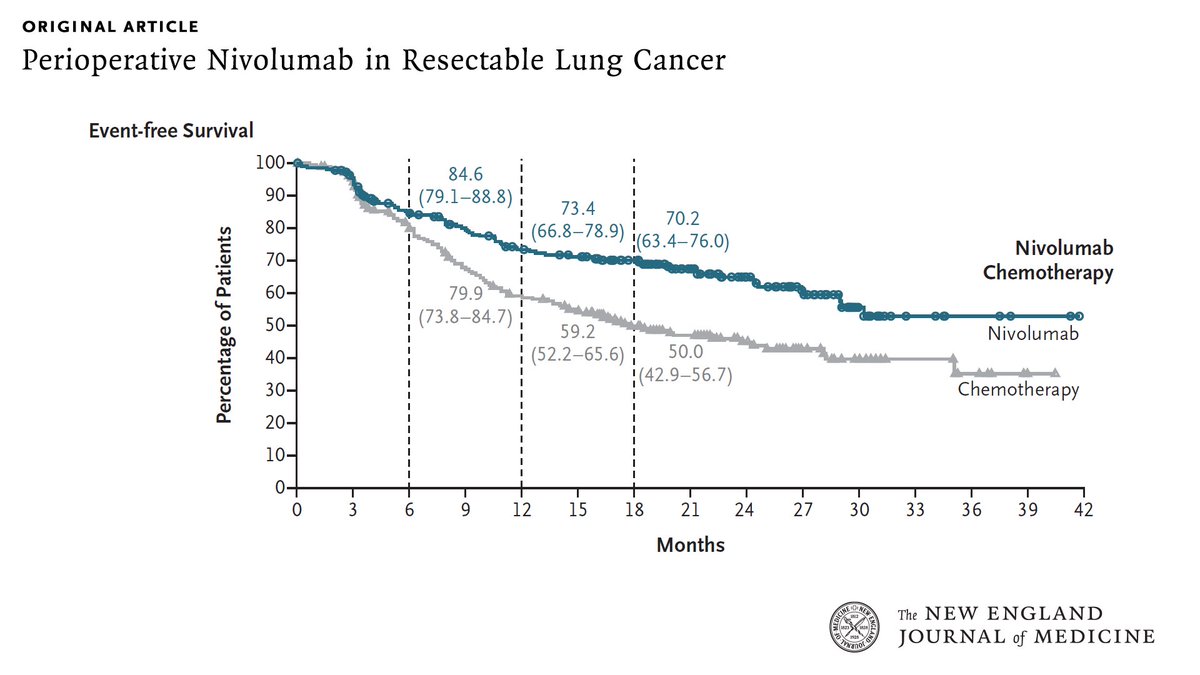
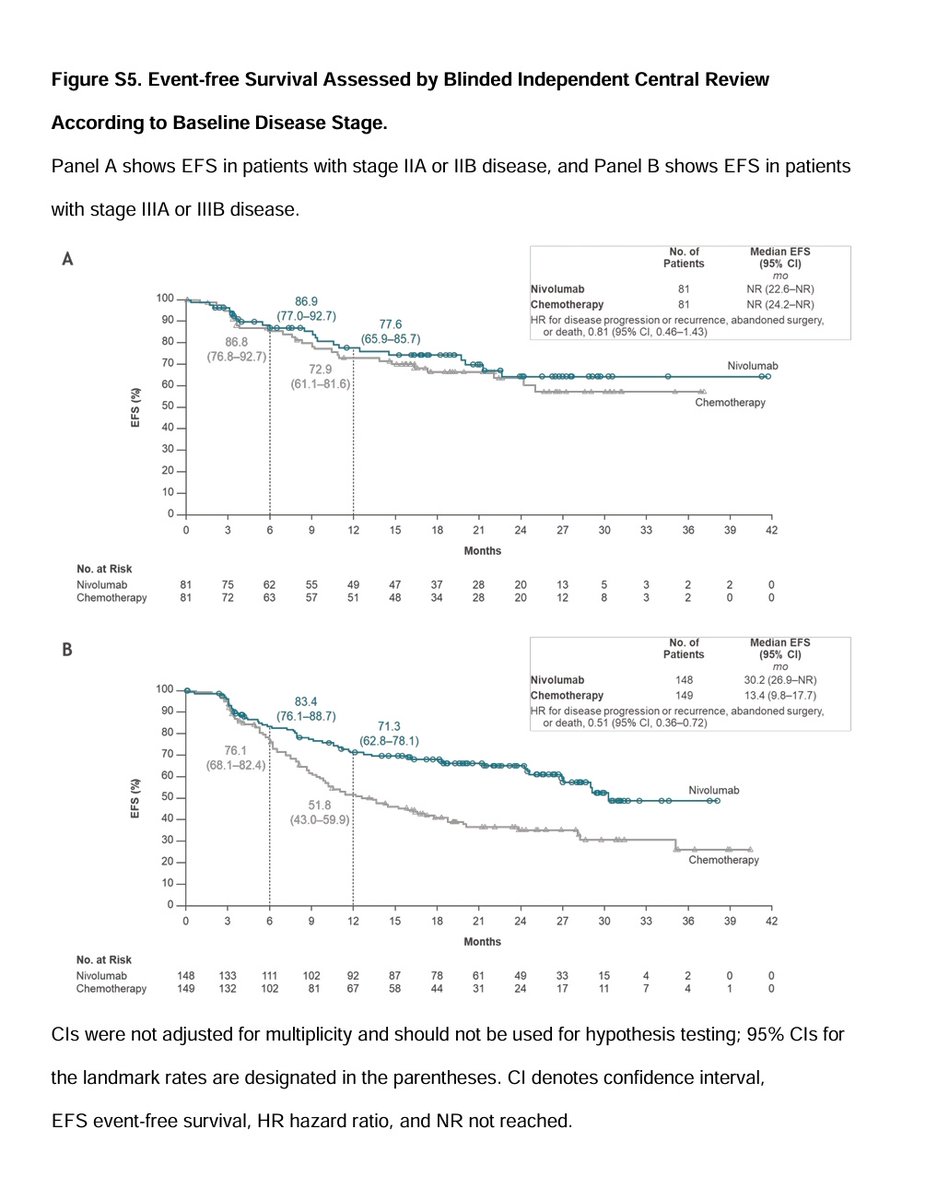
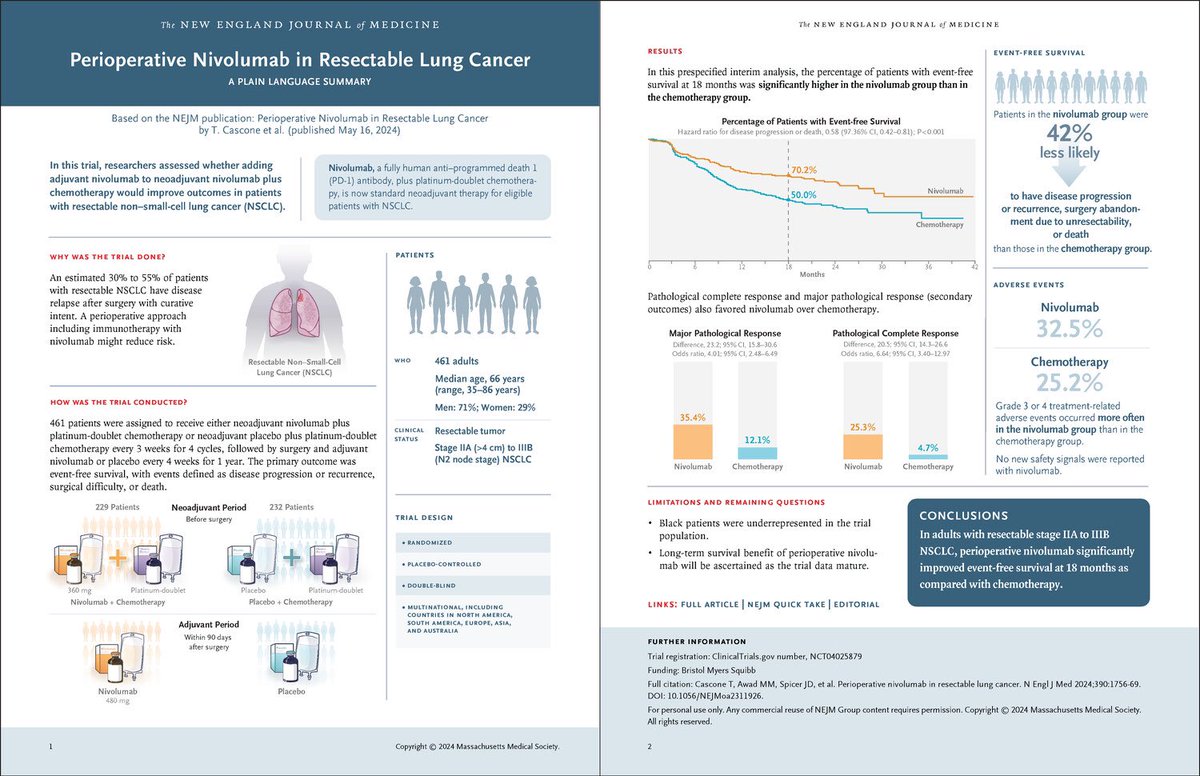
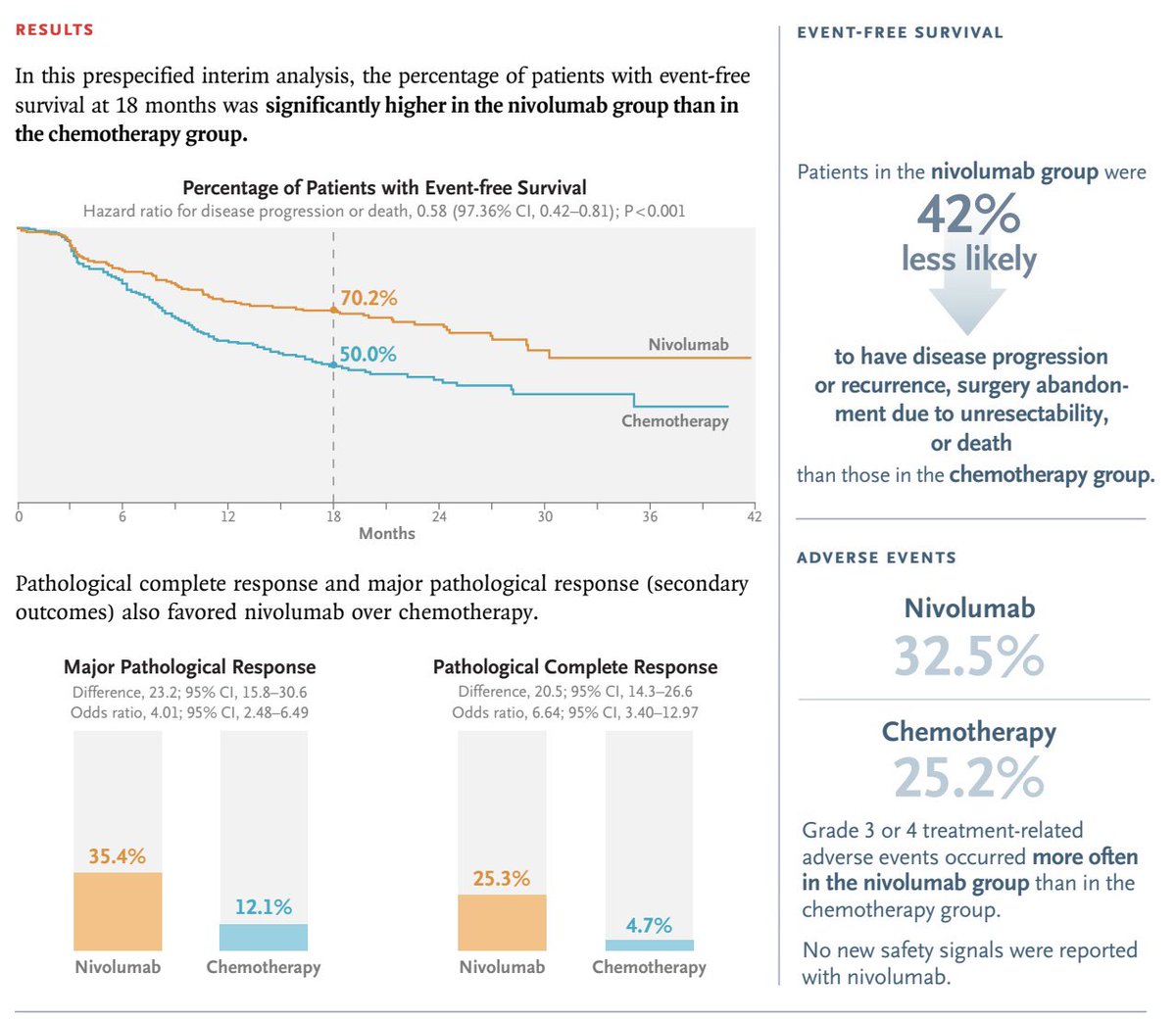
Perioperative nivolumab for resectable NSCLC improves 18-month event-free survival to 70.2% vs. 50.0% with chemo and achieves a 25.3% pathological complete response rate. #LungCancer #Immunotherapy #NSCLC NEJM OncoAlert




Pictures always help!
Just how good is $CRVS interim data for its Phase 2 #A2AR antagonist #Ciforadenant data in combo w/ #Nivolumab + #Ipilimumab ? Really good!
It is at least a 48% deep response rate, which means even higher! Market is not pricing this
nejm.org/doi/full/10.10…
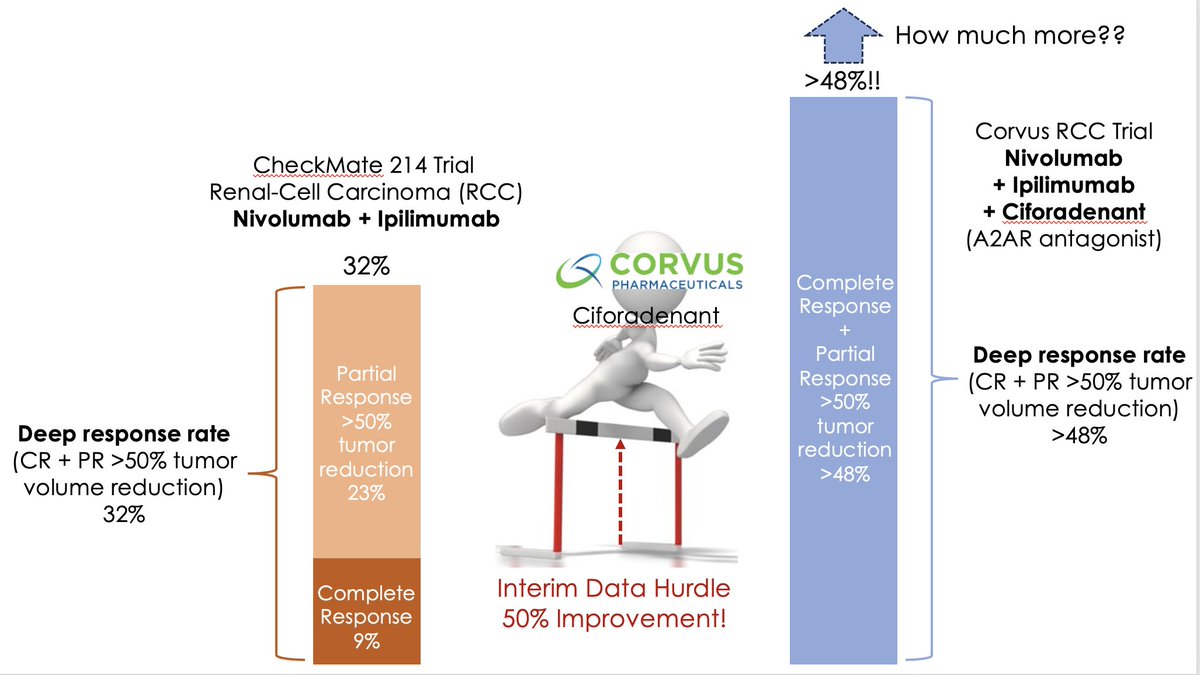

#A2AR antagonists 'Next Generation Checkpoint Blockade for Cancer Immunotherapy' right combo been elusive, until now!
$CRVS combo #Ciforadenant + anti-PD-1 #nivolumab + anti-CTLA-4 #ipilimumab in front-line #RCC >50% more deep responses!
Checkpoint inhibitors growing to $189B!


First-Line Nivolumab/Ipilimumab Improves Survival in Hepatocellular Cancer vist.ly/zkje #livercancer news #livercancer #liverdisease #researchstudy

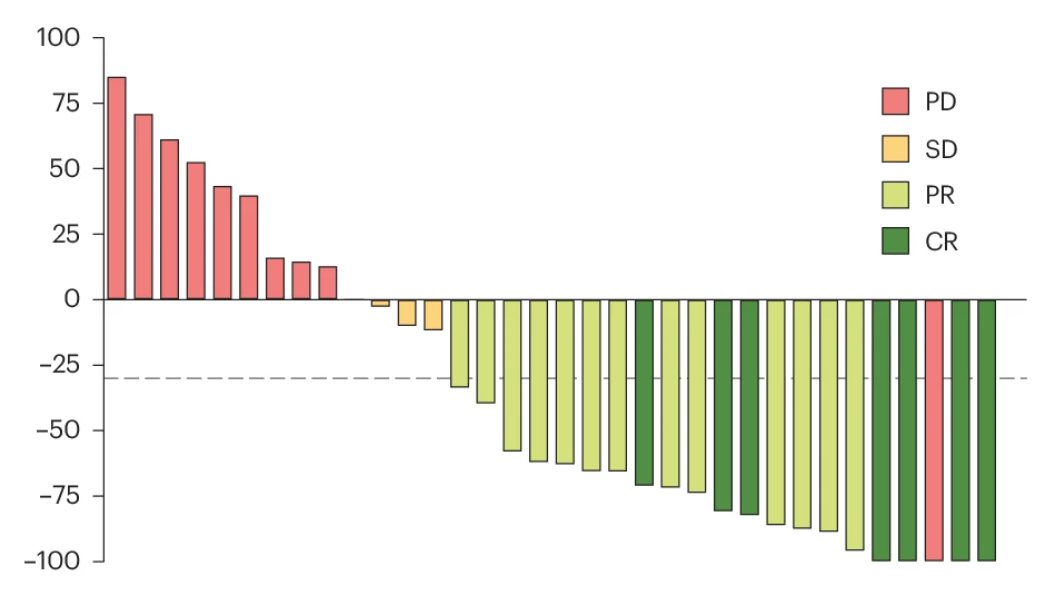
Results of #Checkmate 77T trial are out..
#Perioperative #Nivolumab (neoadjuvant nivo + chemo followed by surgery followed by adj. nivo) in stage IIA-IIIB #NSCLC showing:
⬆️ #DFS & ⬆️ #PCR
And 25% PCR
NEJM OncoAlert Oncology Brothers
iasmintira
#lungcancer
nej.md/3VaMtbX




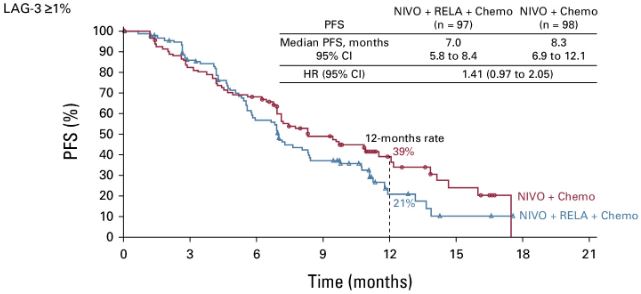
💉Adding relatlimab to nivolumab+ ChT in first line treatment of advanced gastric and GEJ cancer
RELATIVITY-060 Journal of Clinical Oncology
❌Negative study
➡️ORR: 48% vs 61%
➡️mPFS: 7.0 vs 8.3 mo, HR: 1.41 (0.97 to 2.05)
➡️mOS: 13.5 vs 16.0 mo, HR, 1.04: (0.70 to 1.54)




44 months extended follow up of checkmate 9ER trial for advanced RCC . Maximum benifit in poor risk category. No OS benifit in favorable risk category. Intresting to note subsequent therapies in those who completed 2 years of Nivolumab. ESMO - Eur. Oncology Tom Powles

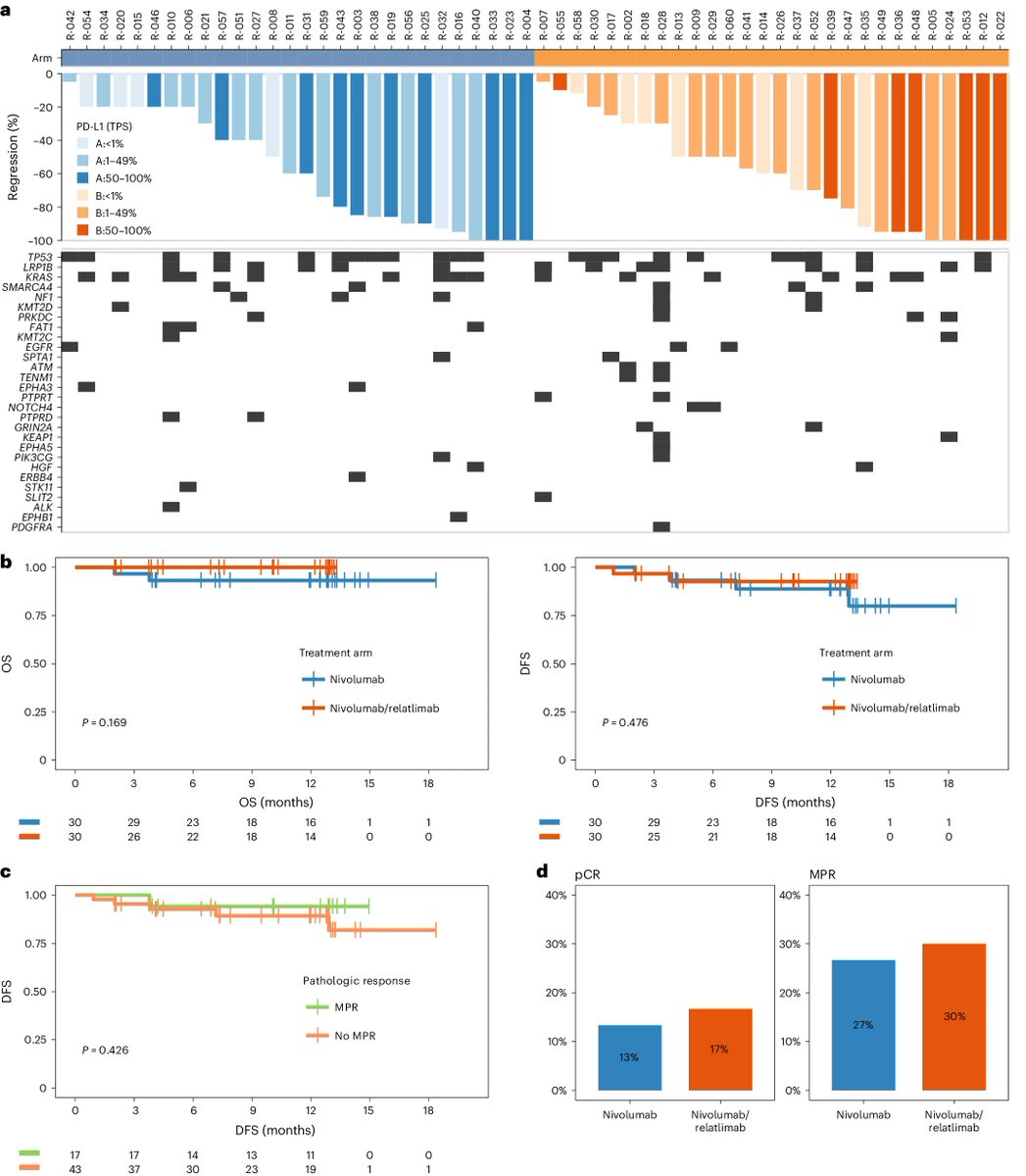
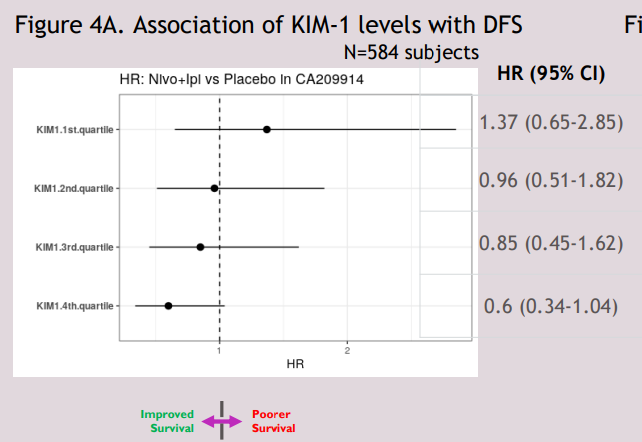
Terrific work! You have been so dedicated to the KIM-1 story, including your recent terrific poster at AACR describing the biomarker in the adjuvant setting via #CM914 ( #nivolumab / #ipilimumab in high-risk localized #kidneycancer ). Proud of you for leading this large at important


Check out our new story out in Nature Medicine today: a phase II study of nivolumab in dMMR/hypermutated gynecologic cancers together with my dear friend Claire Friedman MD . link.springer.com/article/10.103… brief summary (thread):