Jozefina Casuscelli
@jcasusce
MD, Department of Urology, Ludwig-Maximilians-University
ID: 855801726401732609
22-04-2017 15:13:26
142 Tweet
147 Followers
121 Following

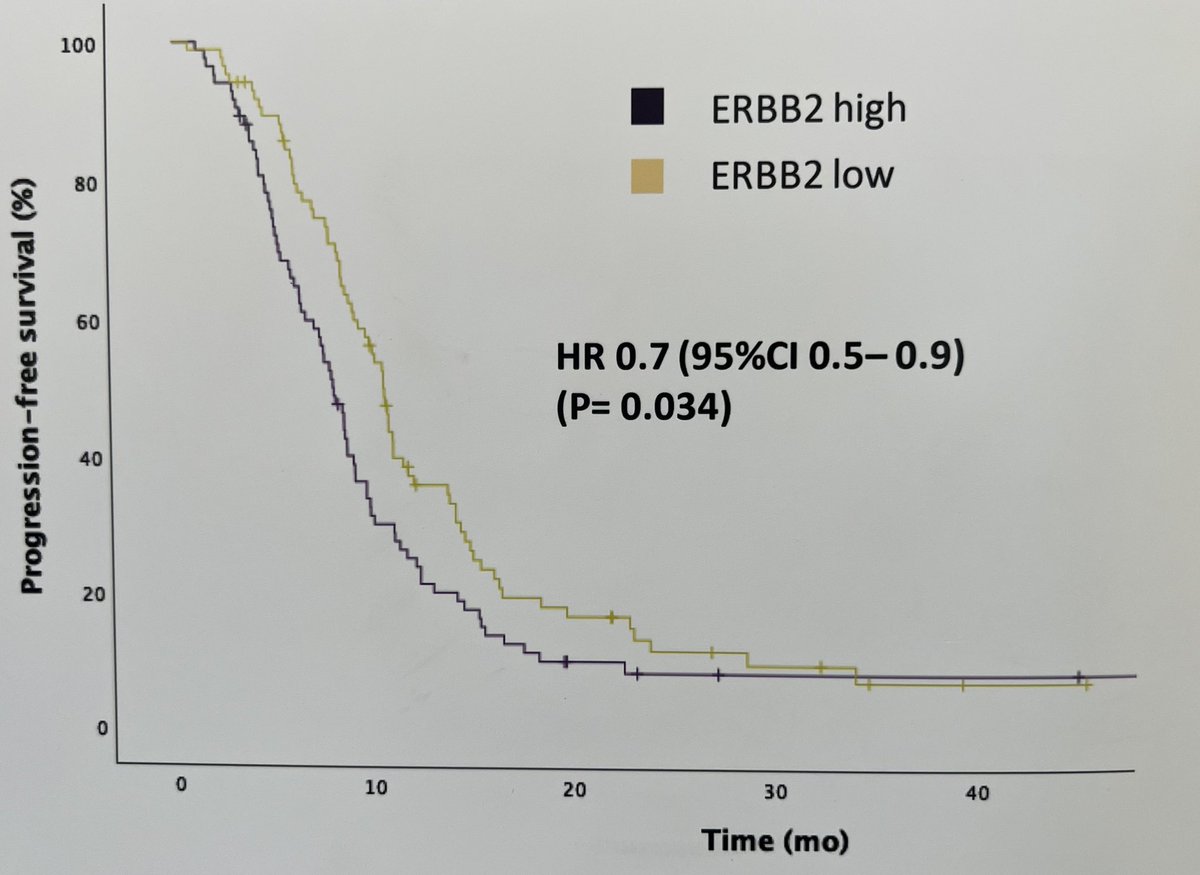
interest in HER 2 & 3 expression in urothelial cancer due to new ADCs. Exploratory analysis from R3 LAMB trial shows ~50% of tumors are HER2 and HER3 +ve. HER2 maybe associated with short PFS. Disitimab vedotin in R3 in 1st line UC, HER3 ADCs awaited in UC Bernadett Szabados @IBCN23

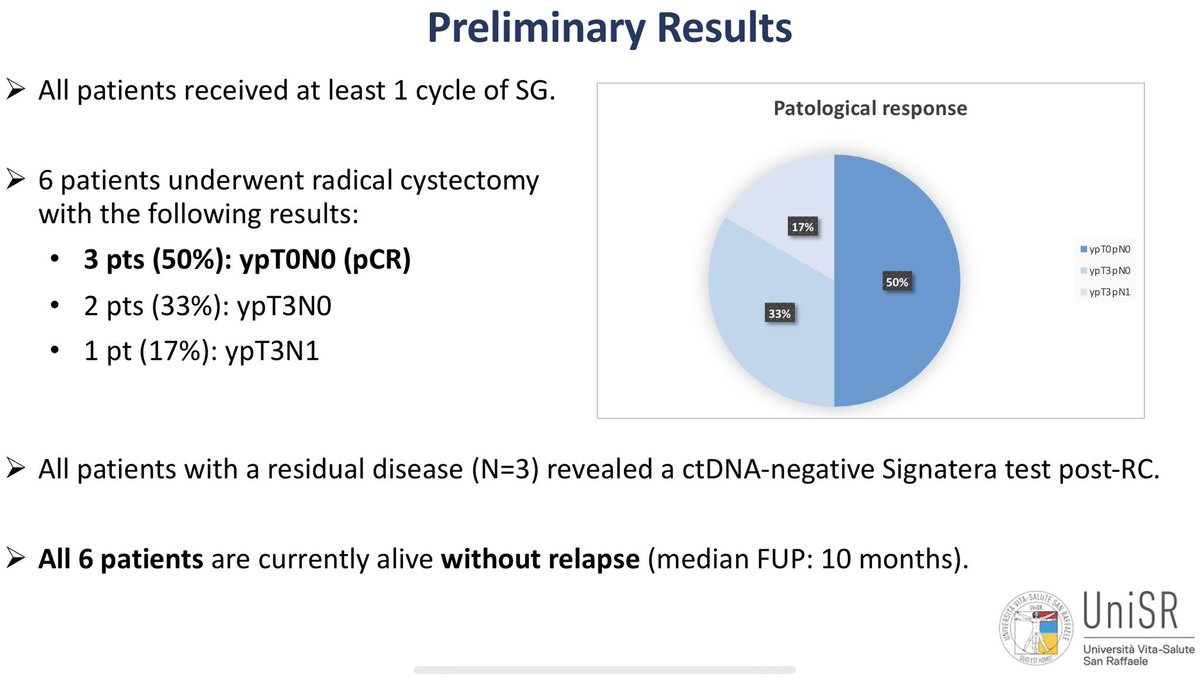
Neoadjuvant sasituzimab govitecan in muscle invasive bladder cancer (n=14) Antonio Cigliola Andrea Necchi @IBCN23 Results = a mixture of efficacy (3/6 pCRs) and toxicity (TRAE G5= GCSF/dose changes). Still early data. EV (n=22, 34% pCR, also with neoadjuvant tox). R3s with EVP

Adjuvant pembrolizumab significance delays disease free survival in bladder cancer. The benefit is ‘clinically meaningful’ Andrea Apolo, M.D. .It joins nivo, but not atezo, in having DFS. It’s not clear whether OS was tested-which is co-primary. Exciting times! merck.com/news/mercks-ke…



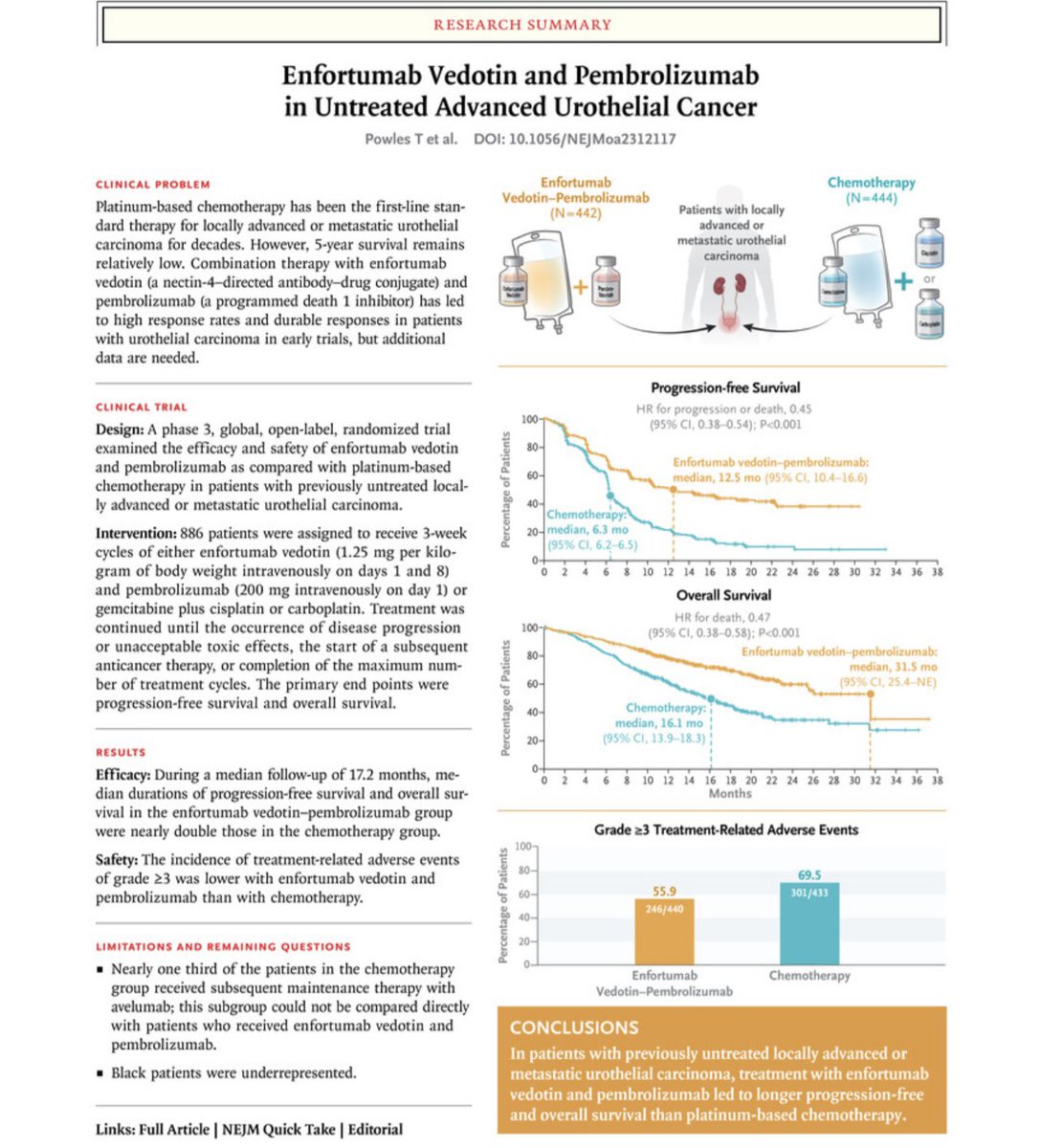
The ESMO - Eur. Oncology LBAs are out And a wow for PEMBRO/EV (Presidential) OS 31.5 vs 16.1 months (HR 0.45) PFS 12.5 vs 6.3 months (HR 0.47) P<0.00001 A historic change for 1L mUC since Von Der Maase JCO 2000 Tom Powles #ESMO23 OncoAlert



Stellar presentation of ABACUS-2 data by Bernadett Szabados: neoadjuvant Atezolizumab as option for sarcomatoid bladder cancer #ESMO23

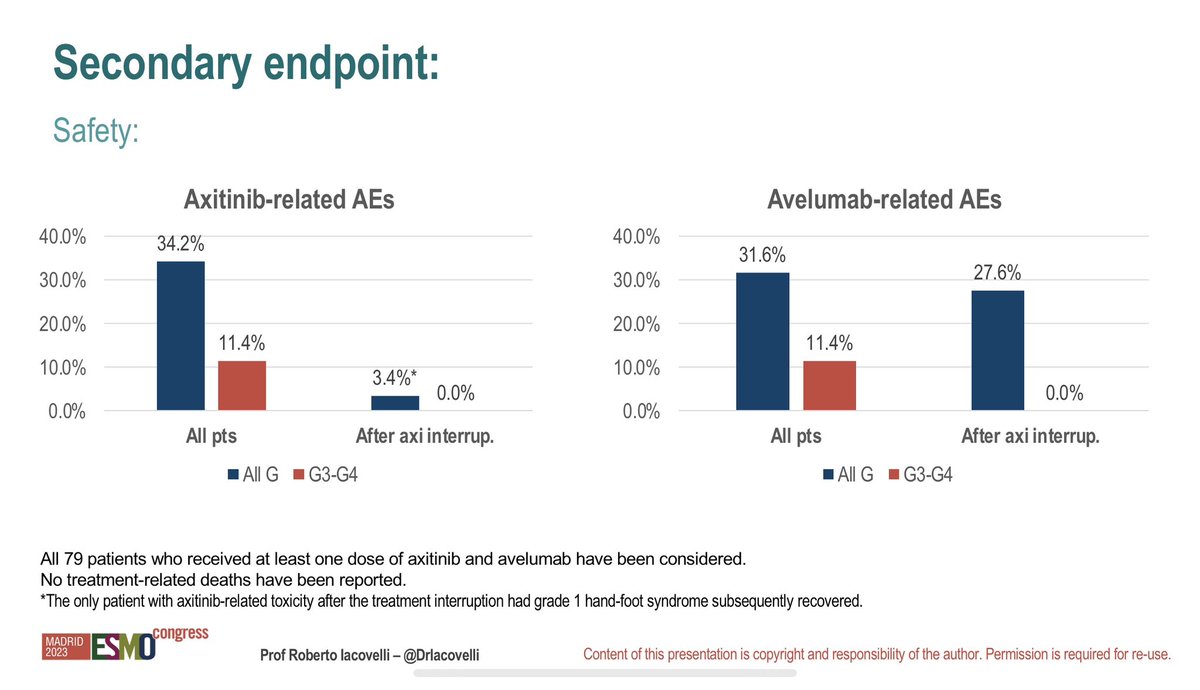
Stopping axitinib after 9 months of axititinib/avelumab in RCC shows median duration of time to progression is only 4 months off therapy #ESMO23 . That’s short and suggests it’s too early to stop? The timing of discontinuation of of therapy in RCC is a key issue. Roberto Iacovelli

Rechallange with pembrolizumab in UC in those patients who complete 2 yrs of therapy or stop due to CR is associated with responses. A highly selected population. What does this mean in the adjuvant therapy setting? #nashvillelive Vadim Koshkin MD #ESMO2023


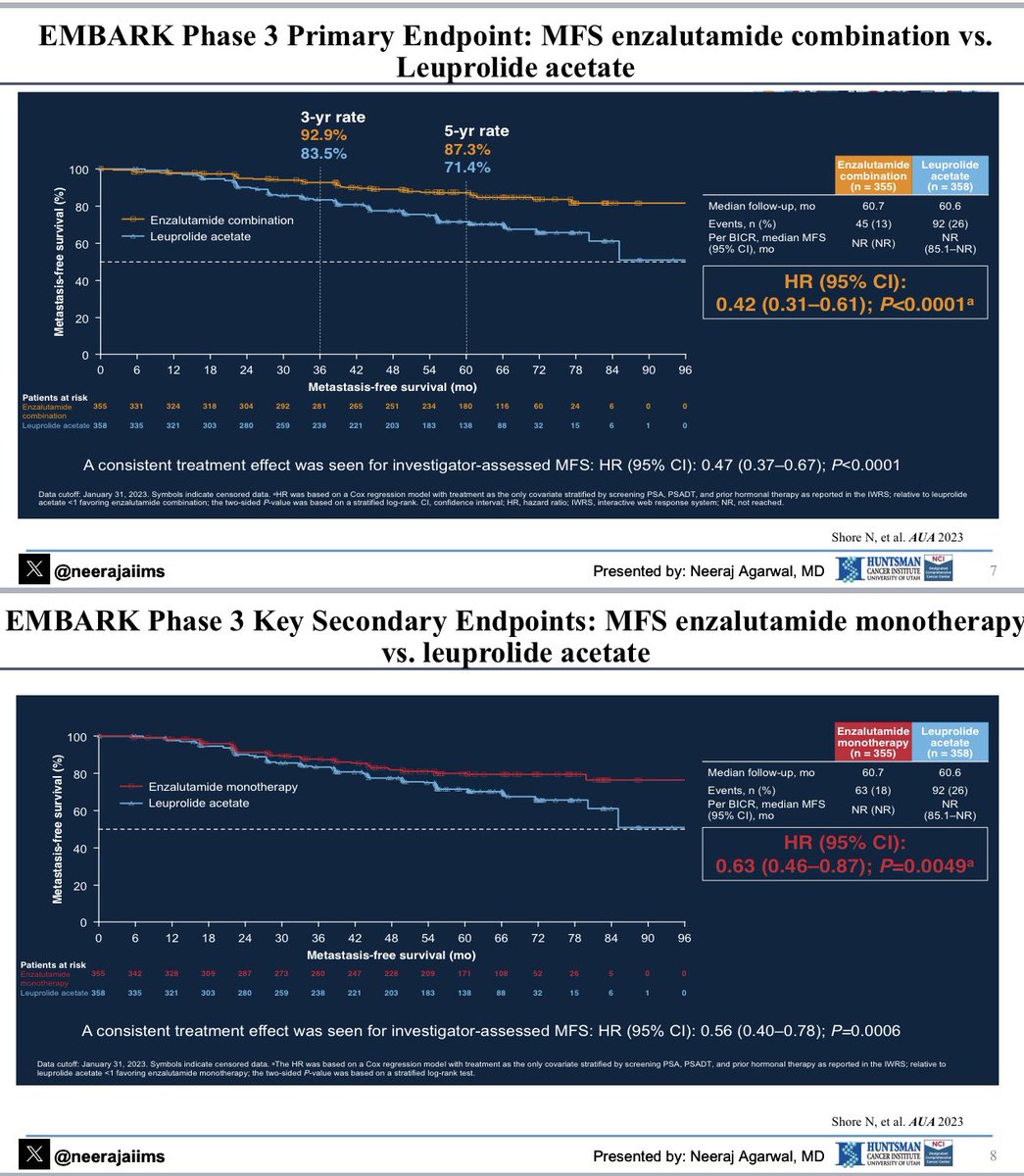
Breaking news👉 U.S. FDA approves enzalutamide with/without androgen deprivation therapy (ADT) for non-metastatic castration sensitive #ProstateCancer based on Embark trial data👇Congrats neal shore Stephen Freedland, MD Link👉 t.ly/R42C6 Prostate Cancer Foundation OncoAlert UroToday.com


JUST IN and again via ASCO #FDAAlerts The FDA Oncology approved Enfortumab Vedotin ( EV) in combo with pembrolizumab for metastatic urothelial cancer!! Data based on Tom Powles #ESMO23 Plenary (EV-302) #StandingOvation ESMO - Eur. Oncology Great day for Bladder Cancer and our


NECTIN4 amplification predicts response to Enfortumab vedotin in mUC! It occurs frequently across solid tumors (app. 25% of mUC)➡️potential tumor-agnostic biomarker for NECTIN4-targeting therapies! Happy to share our poster K17 ASCO #GU24! Markus Eckstein OncoAlert UroToday.com




JUST IN: U.S. FDA alert: FDA approves nivolumab in combination with cisplatin and gemcitabine for unresectable or metastatic urothelial carcinoma! Via ASCO U.S. FDA alert ! Trial presented ESMO - Eur. Oncology #ESMO23 and published NEJM OncoAlert brando Guru P. Sonpavde, MD Michiel van der Heijden


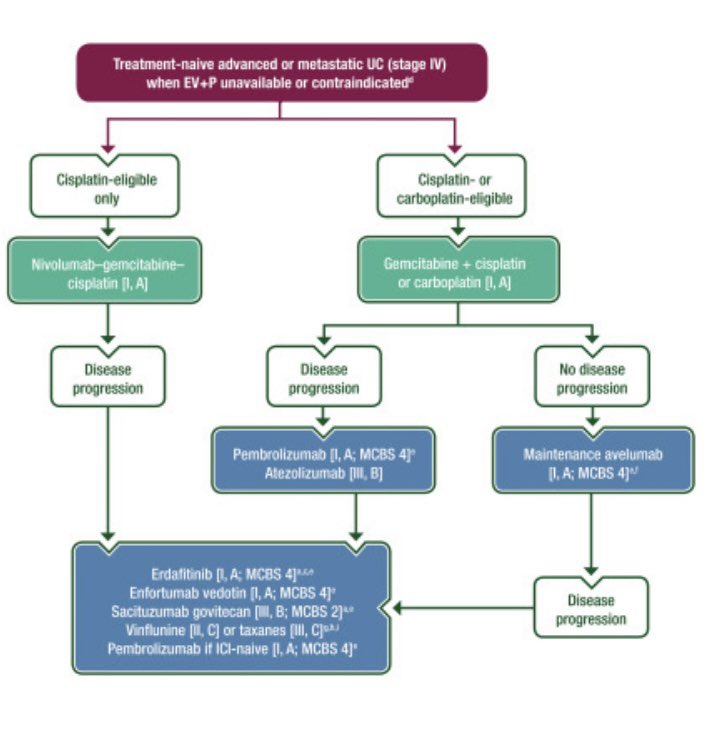
Oncology Brothers Karine Tawagi MD Sia Daneshmand, M.D. CancerNetwork® Bijoy Telivala Yakup Ergün Andrea Apolo, M.D. Santhosh Ambika Amanda Nizam, MD Petros Grivas Shilpa Gupta Enrique Grande Paulo G Bergerot The ESMO guidlines recommends EVP as the preferred standard of care, while nivo/cis and maintenance avelumab are interchangeable and recommended when EVP is not available or contraindicated

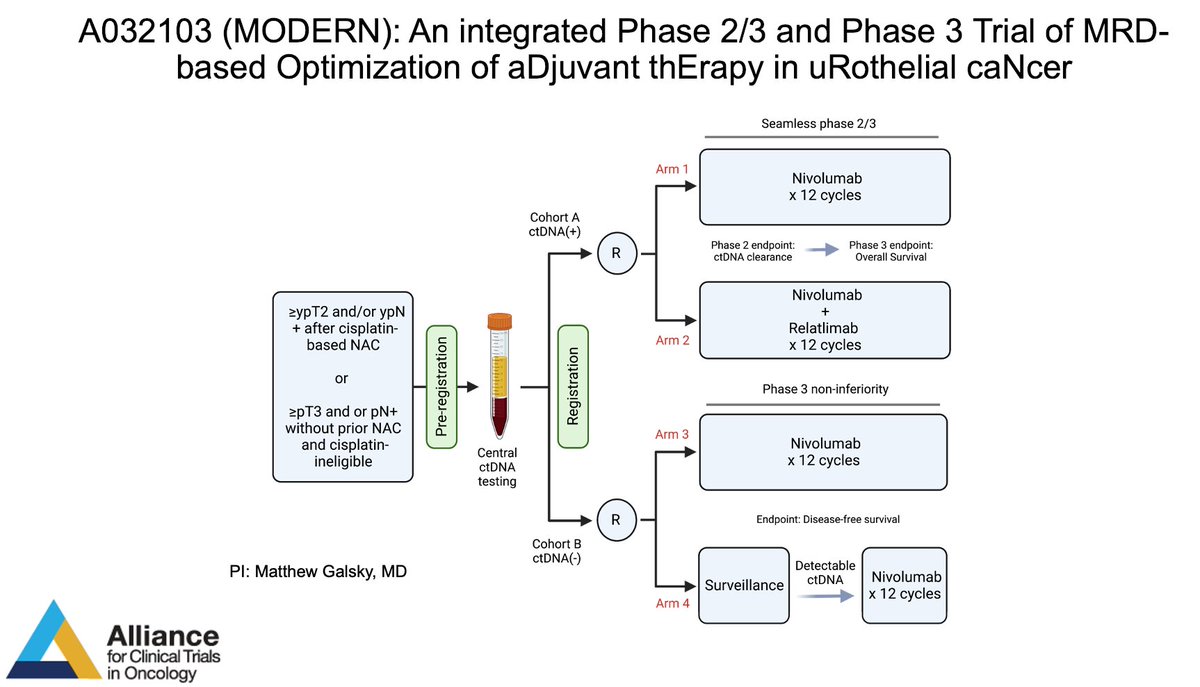
MODERN is open! Available at your site through the CRSU. Please help advance MRD-based adjuvant decision making in bladder cancer. Alliance for Clinical Trials in Oncology Alan Tan Ashish M. Kamat, MD, MBBS ECOG-ACRIN Cancer Research Group SWOG Cancer Research Network Guru P. Sonpavde, MD PaulCrispenMD Stephanie Berg Suzanne Cole, MD, FACP, FASCO Nitin Yerram M.D. DrTylerStewart Jonathan Rosenberg MD