
Marita Lykka MD
@marilykmd
Medical Oncologist- GU cancer Clinical Trials -Deputy Director 4th Oncology Department/ Clinical trials Unit - Henry Dunant Hospital Center
ID: 3372637846
12-07-2015 17:17:58
483 Tweet
96 Followers
138 Following

A huge milestone in #kidneycancer therapy! Hats off to Toni Choueiri, MD Tom Powles & team for leading this pivotal study - & many thanks to the thousands of patients who have participated in adjuvant trials (this & so many others) over the past several decades. Without their courage

The 7th National Hellenic oncology Congress ΕΟΠΕ just began , all of us and our special distinguished guest expert and amazing person Charu Aggarwal, MD, MPH, FASCO are looking forward to welcome all of you!! #some #lcsm Marita Lykka MD ESMO - Eur. Oncology IASLC ASCO OncoAlert


Terrific 7th Annual Hellenic Society of Oncology meeting and a wonderful opportunity to connect with colleagues Giannis Mountzios Helena Linardou Marita Lykka MD - humbled to have contributed to keynote and mentoring sessions and a chance to visit this beautiful country! 🇬🇷 ESMO - Eur. Oncology


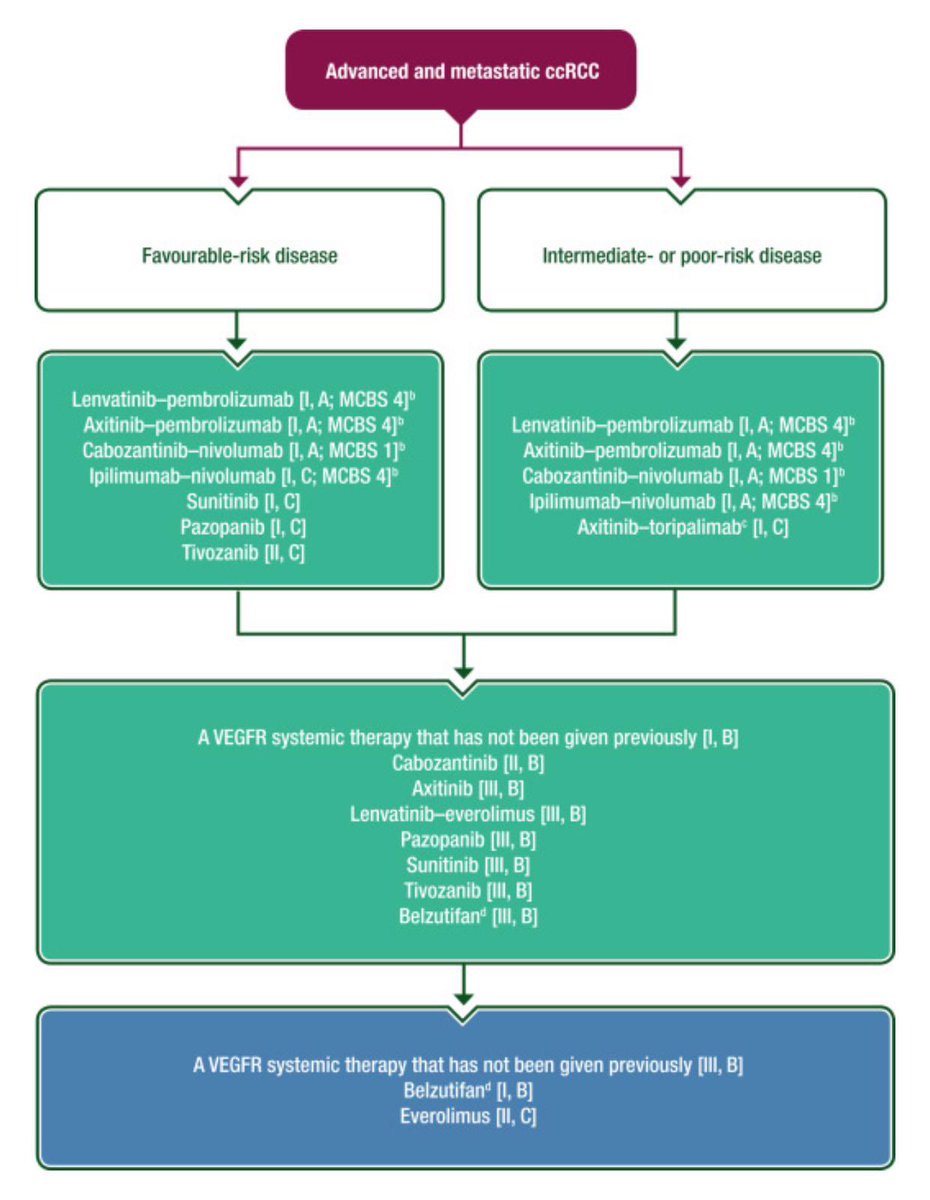
Updated clear cell renal cancer guidlines ESMO - Eur. Oncology.Ipi/nivo now has additional weak support in IMDC good risk (I,C). Belzutifan gets strong support in 3rd line (I,B) but less strong 2nd line (VEGF TKI preferred).1st line PD1/VEGF unchanged Annals of Oncology ow.ly/nZA050RP77l

Join us for fantastic meeting in Vienna on practical data applications in GU cancers! Giannis Mountzios Marinos Tsiatas Marita Lykka MD MikaLion Ζenia Saridaki Enrique Grande Daniel Castellano Bernadett Szabados Uromigos Ursula Vogl Badrinath Konety, MD, MBA Vadim Koshkin MD silke gillessen OncoAlert Myrto K. Moutafi ANASTASIOS KYRIAZOGLOU


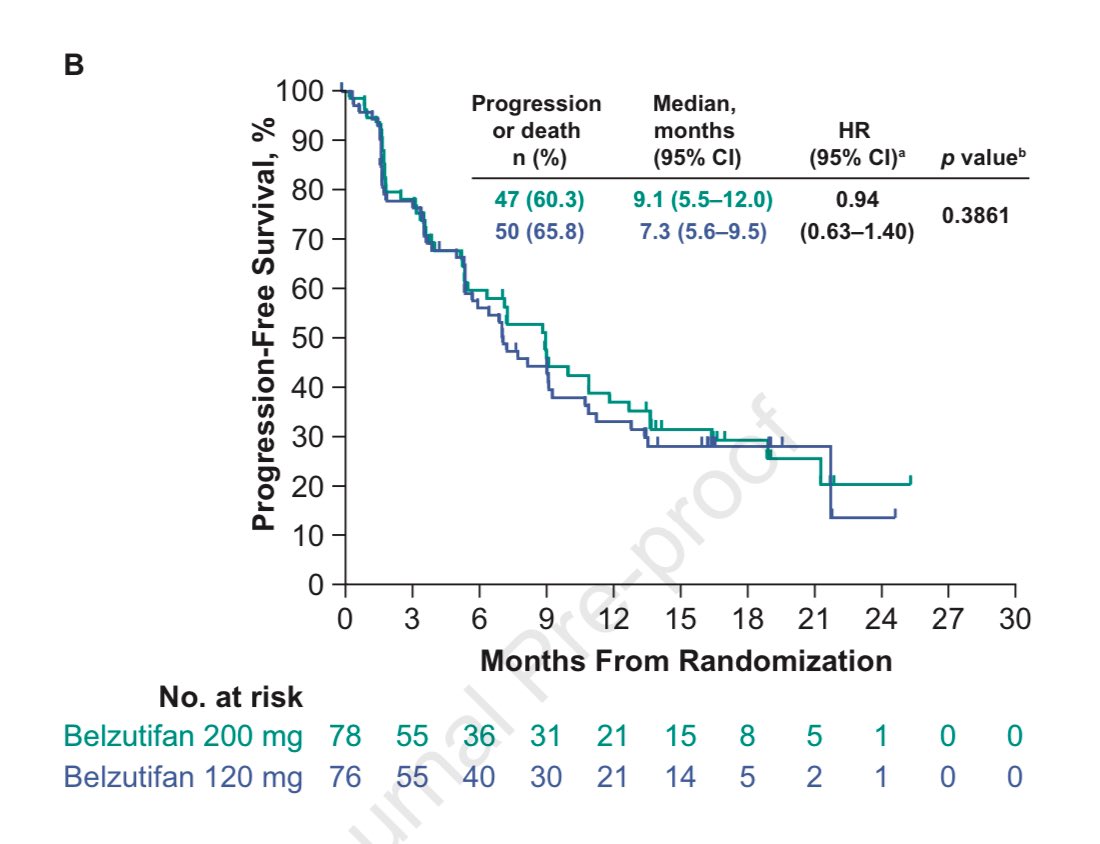
Just in Annals of Oncology 👉on the heels of the recent approval of belzutifan in mRCC #kidneycancer , herein we show in a ph2 randomized trial👉more belzutifan (200 mg vs 120 mg QD) was not better👇Link: tinyurl.com/nrsxp8s4 UroToday.com OncoAlert KidneyCAN Huntsman Cancer Institute


📰 If you found these facts of interest, stay tuned for an upcoming review article led by Raffaele Colombo & myself, which will retrace the lessons learned over the last 40 years of development of ADCs Also, make sure to attend the ADC session of #ENA24! event.eortc.org/ena2024 11/11



Exciting 177Lu-PSMA-617 has been approved by the FDA for use prior to chemotherapy based on the PSMAfore results. Perfect timing, during day 1 of the PSMA and Beyond Conference! bit.ly/42lfdlz




Join our friends & 🚨Faculty Brian Rini, MD 🇺🇸and Tom Powles 🇬🇧 (The Uromigos ) as they discuss: ADC 💊#BladderCancer PART 2⃣ New ADCs in bladder cancer THIS IS PART TWO IN A TWO PART SERIES Sumanta K. Pal, MD, FASCO Cristiane D Bergerot Daniel Heng Andrea Apolo, M.D. Toni Choueiri, MD Petros Grivas



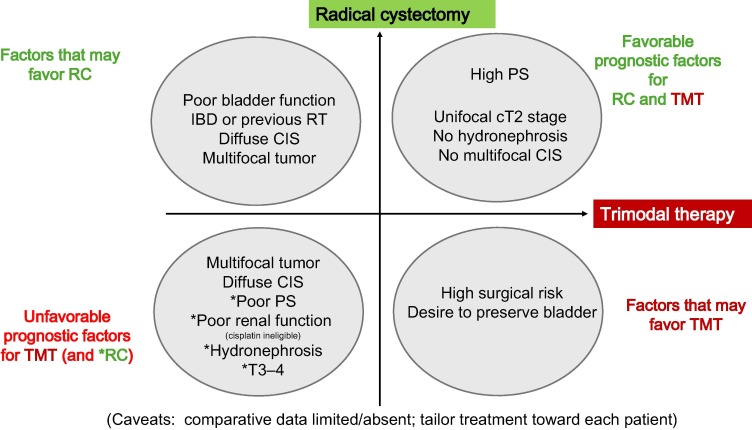
⚡️ In European Urology - Bladder Preservation Strategies in Muscle-invasive Bladder Cancer: Recommendations from the International Bladder Cancer Group #BladderCancer Ashish M. Kamat, MD, MBBS Shilpa Gupta Prof Ananya💙 @achoud72.bsky.social Dr. Bishoy M. Faltas Kent Mouw Andrea Necchi Petros Grivas Roger Li

Final MAGNITUDE 📢 In mCRPC with BRCA mutations: Niraparib to Abiraterone = ⬇️ progression risk by 47% ⏳ rPFS: 19.5 vs 10.9 months 🧬 No benefit in non-HRR pts ✅ Confirmed the biomarker-driven benefit! European Urology Oncology OncoAlert Advanced Prostate Cancer Consensus Conference Elena Castro AttardLab








