Cal Patel
@calumpatel
University of Oxford Chemistry PhD Candidate - @GouverneurGroup - he/him
ID: 386534098
07-10-2011 13:10:10
2,2K Tweet
334 Takipçi
674 Takip Edilen


We can't wait to hear Oxford Chemistry DPhil Cal Patel speak at our Autumn Chemistry Conference (26th Sept, 16:00-18:00 BST) about his research in the Gouverneur Group that led to a breakthrough in fluorine chemistry. Want a sneak preview? Check out: youtube.com/watch?v=9kgPmX…


Check out our latest work on the radiosynthesis of 18F-difluoromethyl(ene) motifs online now in Org Lett J Org Chem/Org Lett! Congrats to the team! ☢️ pubs.acs.org/doi/10.1021/ac…

Thrilled to share our new study of anti-Bredt olefins in Science Magazine. Congrats to the authors for this nicely timed study, 100 yrs after “Bredt’s Rule” came to be. Read it here: science.org/doi/10.1126/sc… @ucla UCLA Chem & Biochem Ken Houk

Just published in nature, our paper on mobile robots for exploratory synthesis: nature.com/articles/s4158… Chemistry News Chemistry World Nature Chemistry Royal Society of Chemistry American Chemical Society MIF University of Liverpool Leverhulme Research Centre - Materials Design A I Cooper group



A hexavalent nickel complex! Reductive addition of three Be-Be bonds at Ni(0) produces Ni(BeCp)6. Aldridge Group pubs.acs.org/doi/10.1021/ja…




Delighted to share our latest research on a simple coupling reaction of two strained diradicaloids, just published in nature. Congrats to Arismel Tena Meza, Christina, Huiling Shao, and Andrew, plus our amazing collaborator @Houk1000! PDF: rdcu.be/d9Chn UCLA UCLA Chem & Biochem


Thrilled to share our newest work on the enantioconvergent synthesis of alkyl fluorides with KF, made possible by a synergistic combination of chiral urea and onium salt catalysts. Out today in Nature Catalysis doi.org/10.1038/s41929… Congrats to Claire Dooley and all of the team!

Delighted to see my PhD research published today in Nature Catalysis! Here we disclose the latest work in our hydrogen bonding research programme, synthesising alkyl fluorides using KF under synergistic phase transfer catalysis. Thanks to all involved who made this possible 🎉


Interested to learn more about furans! check out our newest J. Am. Chem. Soc. Nils Frank pubs.acs.org/doi/10.1021/ja…


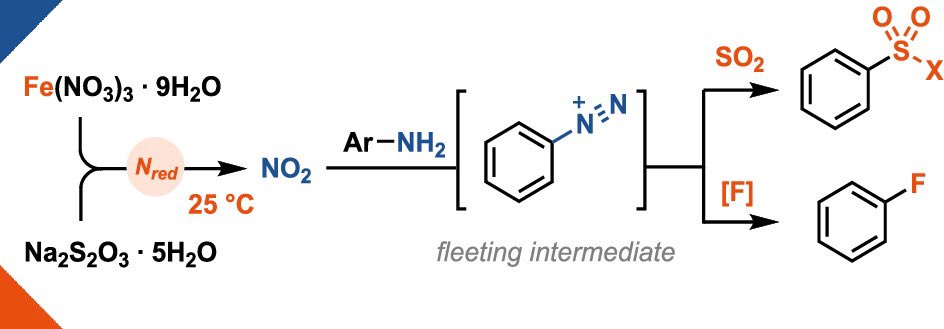
Nitrate reduction at 25 °C? A little iron is all it takes! 🪄 The use of cheap iron(III) nitrate (30€ per kg!) enables the in-situ generation of aryldiazonium salts which can be engaged in sulfonylation & fluorination reactions! Out now in J. Am. Chem. Soc. 🔓 pubs.acs.org/doi/10.1021/ja…