Sandeep Kumar Thakur
@sandeepthakur_k
PostDoc @Harder_research @UniFAU 🇩🇪
Ph.D @IISERMohali 🇮🇳 with @singhlabIISERM
Research Interest:Modern carbenes (CAAC/BICAAC); An Organometallic chemist.
ID: 1404410701259542530
https://www.inorgchem1.nat.fau.de/ 14-06-2021 12:10:03
198 Tweet
270 Followers
439 Following

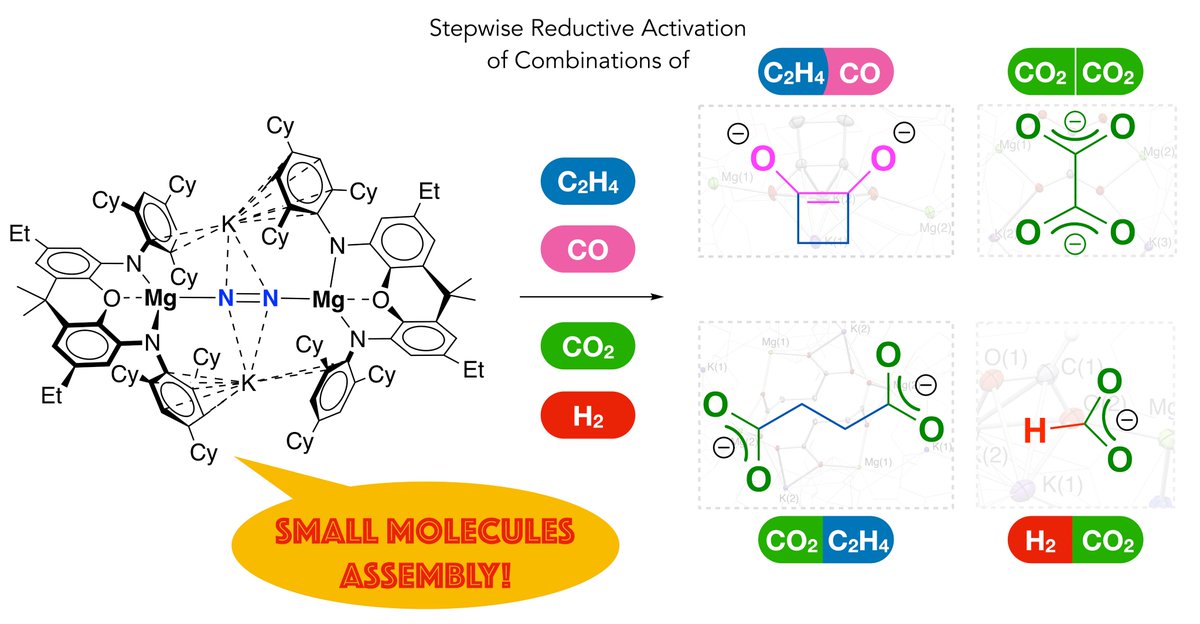
Dat (Dat Tien Nguyen) and Rahul's paper on a Mg-N2 complex inducing cross-couplings of H2, CO, C2H4 and/or CO2, giving functionalised organics, has been accepted Angewandte Chemie tinyurl.com/53rjm38v. Some heroic xtallography by Matthew Evans on this one. #ozchem Monash Chemistry

New paper from Martyn Coles, a very funky Sn4 species 🧪💻

Room-Temperature H2 Splitting and N2-Hydrogenation Induced by a Neutral LuII Complex | Journal of the American Chemical Society Institut Polytechnique de Paris CNRS 🌍 Université de Toulouse pubs.acs.org/doi/10.1021/ja…



This guy is finally out!! Glad to collaborate with Harder-Research and Sandeep Kumar Thakur, and help them describe the first s-Block-Lanthanide bond! Awesome chemistry that you can now check out here in J. Am. Chem. Soc. ALGC 🚀🚀 pubs.acs.org/doi/10.1021/ja…

I am extremely happy to share my second project published in J. Am. Chem. Soc. . Check out our paper to see bonding between two electropositive metals. Huge thanks to Prof Sjoerd Harder for support Harder-Research computational partners Nil Roig Vidal Prof Mercedes.pubs.acs.org/doi/10.1021/ja…


Siad's work (Siad Wolff) on dimanganese(I), and Mn-N2 bonded compounds, which closely mirrors our prior Mg chemistry, has appeared as a preprint ChemRxiv. Part of a nice collab with project leader Christian Limberg tinyurl.com/2ds3es5p #ozchem Monash Chemistry Humboldt Berlin Chemistry


A rare Mg–Yb complex that exemplifies s-block metal-Ln interactions is reported in J. Am. Chem. Soc.. CSD Entry NUQJES confirms major electrostatic Mg0-M2+ bonding and establishes that Yb can act as a 3-electron reductant. 🔗 ccdc-info.com/3E9ypJX #FeaturedStructureFriday


CO2 REDUCTION WITH LOW-VALENT CALCIUM: Ca-N2 complexes react like synthons for low-valent Ca(I). We discuss first intermediates and products in CO2, CS2 and RN=C=NR reduction. ChemistryEur Details on BlueSky tinyurl.com/3vjx83tv






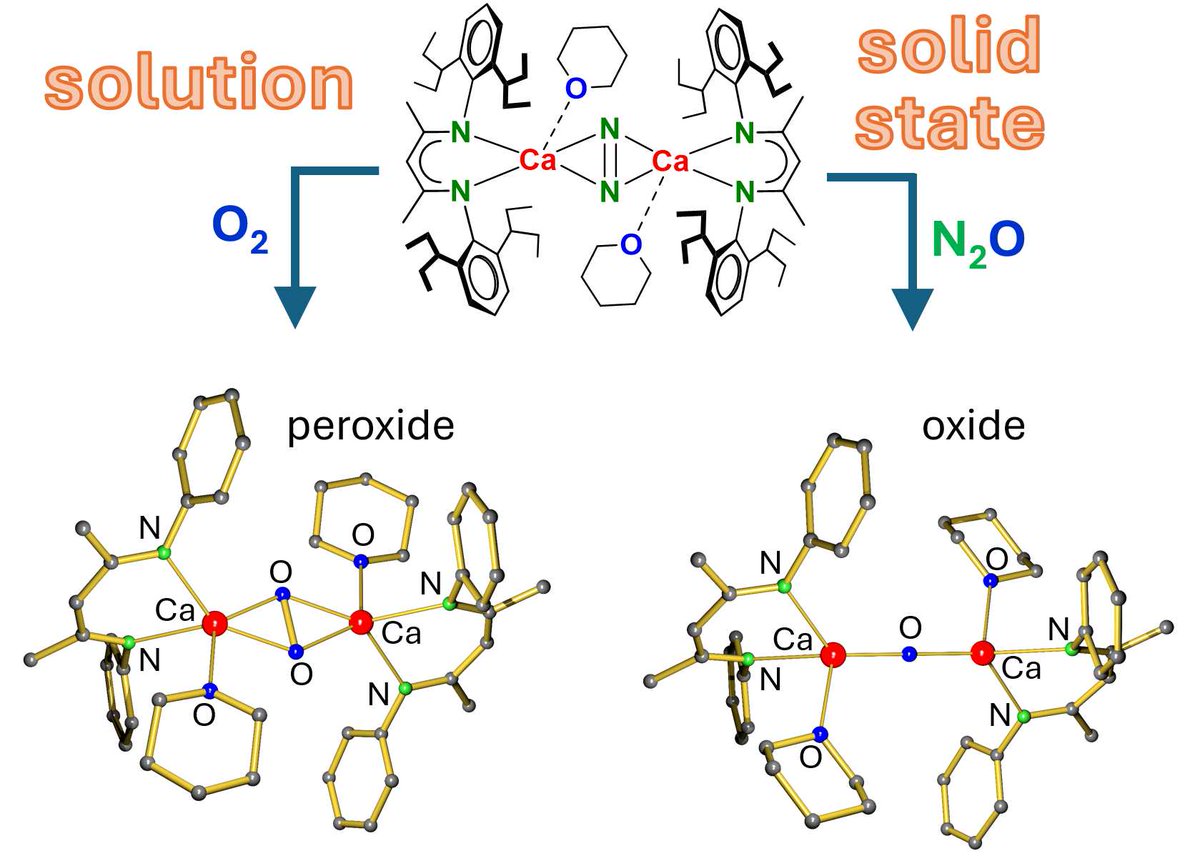
A Ca(I) synthon with bridging N2 is key to the synthesis of homometallic Ca peroxide and oxide complexes. In solution, the Ca oxide complex decomposes even at -80 C. It can only be prepared in the solid state. Chemical Communications Royal Society of Chemistry shorturl.at/AUoWM