FDAAA TrialsTracker
@fdaaatracker
Which trials have not reported their results according to the FDAAA? Find out on the FDAAA TrialsTracker!
A project from @BennettOxford
ID: 966235409041821696
https://fdaaa.trialstracker.net/ 21-02-2018 08:57:28
196 Tweet
512 Takipçi
13 Takip Edilen

JOURNALISTS: We have a very exciting paper on FAILURE TO REPORT CLINICAL TRIAL RESULTS CORRECTLY coming tomorrow FRIDAY, embargoed to 23:30 UK time. If you would like the press release please email [email protected] and cc me [email protected]

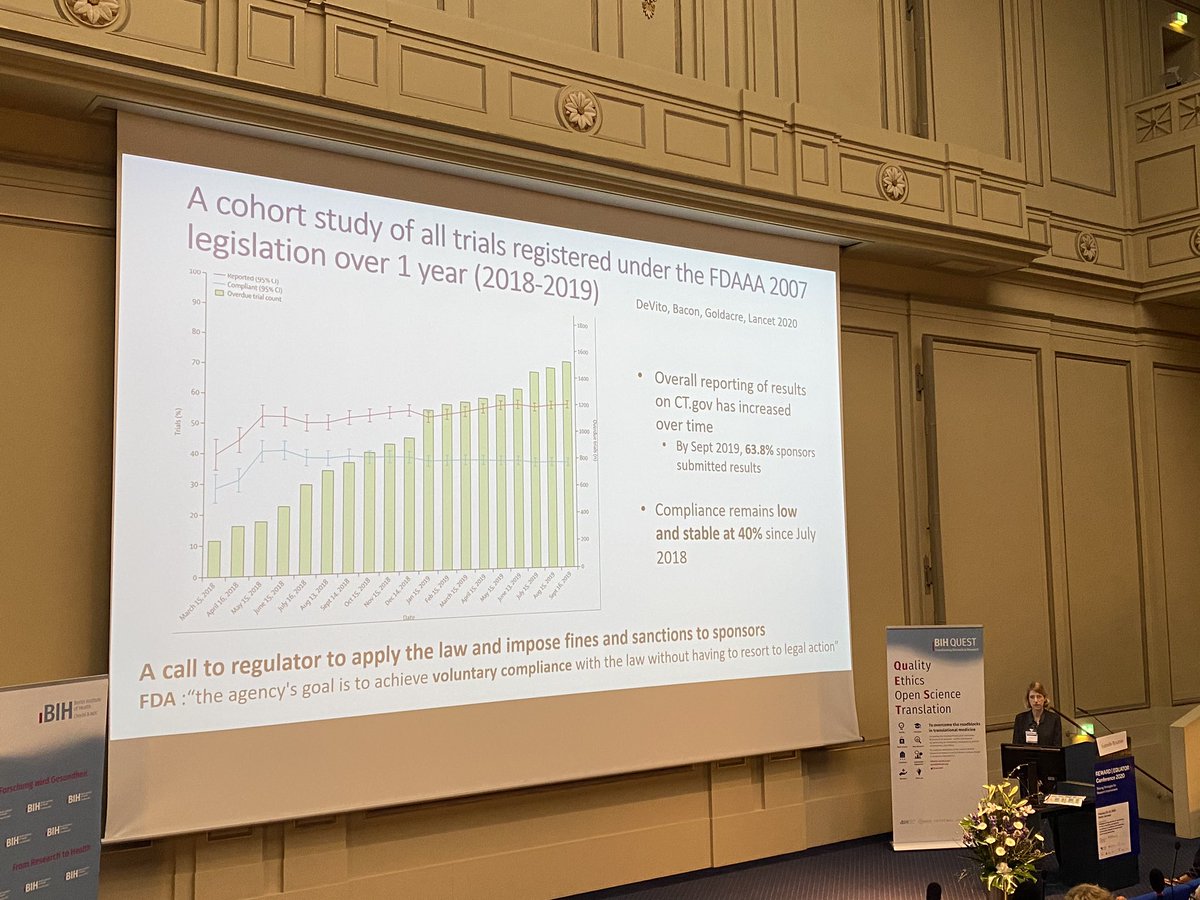
BING BONG NEW PAPER Nick DeVito @[email protected] and me in the LANCET today. We definitively assess compliance with US law FDAAA 2007, which requires clinical trials to report results within 12 months of completion. The answer: 59% of trials breached the law thelancet.com/journals/lance…



Just won (mostly) a court case against @us_fda: agency can no longer exempt certain trial results from reporting on clinicaltrials.gov contrary to law (FDAAA). Thx to co-plaintiff Peter G. Lurie, MD & litigators MFIA Clinic NYU Law @Yale_CRIT. documentcloud.org/documents/6784…



I'll shout more about this when it isn't Friday evening but my preprint on COVID trial reporting with Peter and Maia Salholz-Hillel Daniel Strech from @questbih is up! Check it out for a weekend read!

My group in Oxford has published LOTS on who does, and doesn't, report their clinical trial results. But what about the rest: late registrations, data verification, certificates of delay, document sharing? Our Nick DeVito lashes detail in JAMA Internal Medicine jamanetwork.com/journals/jamai…

Consistent with our findings from the FDAAA TrialsTracker on sponsor size being a major predictor of FDAAA compliance Here: thelancet.com/journals/lance… And here: jamanetwork.com/journals/jamai… And consistent with the EU as well here: bmj.com/content/362/bm…


In news probably only a handful of people care about, but drove me crazy, ClinicalTrials.gov is finally closing a loophole that let people submit delays of results for good cause much later than should be allowed under FDAAA Reshma Ramachandran TranspariMED Deborah Zarin



Come and work with us. This is an unprecedentedly awesome job. Three years with the team that builds OpenSAFELY FDAAA TrialsTracker OpenPrescribing AND Oxford college affiliation, with all the dinners and discursive chats across disciplines that entails!

WE ARE HIRING Junior Research Fellow with college affiliation. That means meals and rich social/intellectual community on top of 3 years with the mighty team that builds OpenSAFELY FDAAA TrialsTracker OpenPrescribing Come change the world through data and tools!

WE ARE HIRING! A prestigious fellowship at Jesus College Oxford working with us on your own projects around epidemiology, open science, research software engineering and all our usual obsessions! Bennett Institute for Applied Data Science OpenSAFELY OpenPrescribing FDAAA TrialsTracker and more bennett.ox.ac.uk/blog/2023/02/w…




We are launching a project on improving code sharing in health and medical research funded by UK Research and Innovation. Read more here: bennett.ox.ac.uk/blog/2025/08/h…