Boron-Chem-Research
@Boron_Chemistry
Latest research in organoboron chemistry
ID:809101410558242816
14-12-2016 18:23:03
4,1K Tweets
18,4K Followers
0 Following

Insights into the Distinct Behaviors between Bifunctional and Binary Organoborane Catalysts through Terpolymerization of Epoxide, CO2, and Anhydride (Angewandte Chemie): onlinelibrary.wiley.com/doi/10.1002/an…


Boron-Catalyzed C1 Copolymerization of Arsonium and Sulfoxonium Ylides toward Unrepresented Structures and Fluorescence Properties (Angewandte Chemie): onlinelibrary.wiley.com/doi/10.1002/an….


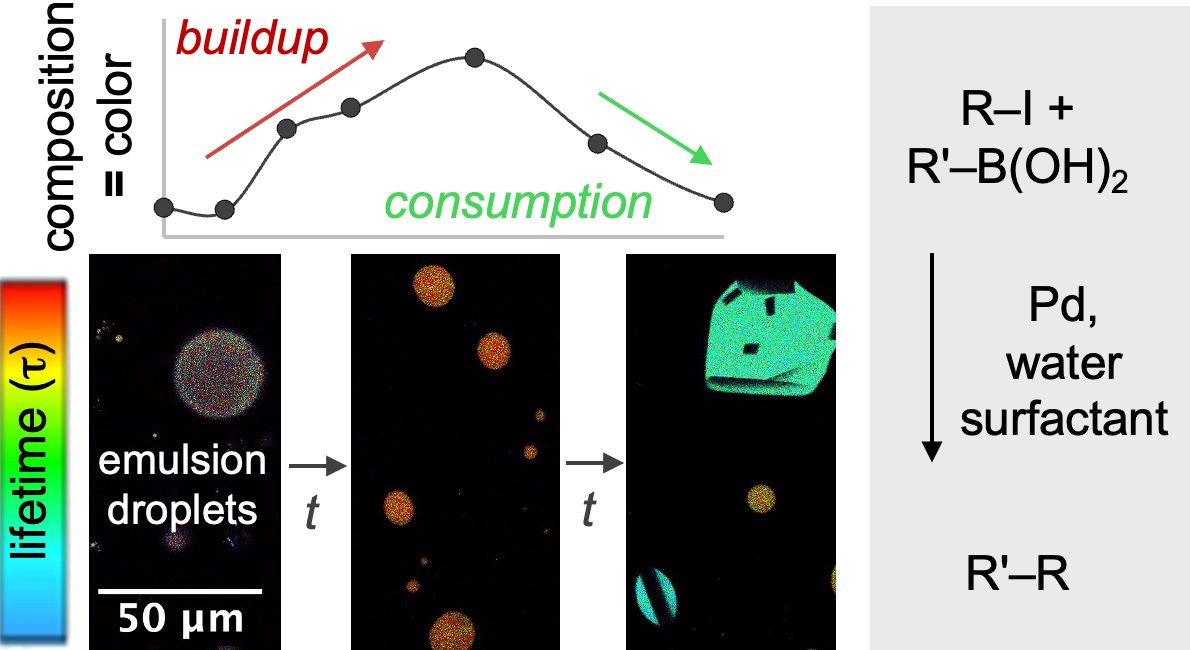
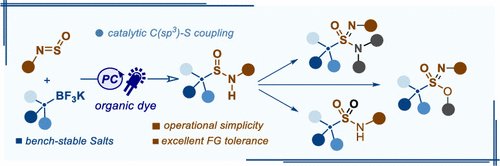
Unifying N-Sulfinylamines with Alkyltrifluoroborates by Organophotoredox Catalysis: Access to Functionalized Alkylsulfinamides and High-Valent S(VI) Analogues (J Org Chem/Org Lett): pubs.acs.org/doi/10.1021/ac….

If you are a prospective #postdoc from anywhere in the world interested in glowy things and their exploitation, consider coming applying for a #MSCA postdoctoral fellowship to work with the EZ-C Group at St Andrews School of Chemistry in #Scotland . If interested, please contact Eli Zysman-Colman.

Herbert C. Brown Lectures in Organic Chemistry ! Purdue Chemistry
😀Impressions with Tom Rovis, Shannon Stahl, Chris Uyeda, students and poster award winners 👏🚀🎉Northwestern Chemistry
Uyeda Lab Rovis Group Stahl Lab Glorius Group
chem.purdue.edu/hcbrownlecture…



NIR-II Absorption/Fluorescence of D–A π-Conjugated Polymers Composed of Strong Electron Acceptors Based on Boron-Fused Azobenzene Complexes (Angewandte Chemie): onlinelibrary.wiley.com/doi/10.1002/an….


Simple and Green Preparation of Tetraalkoxydiborons and Diboron Diolates from Tetrahydroxydiboron (J Org Chem/Org Lett): pubs.acs.org/doi/10.1021/ac….


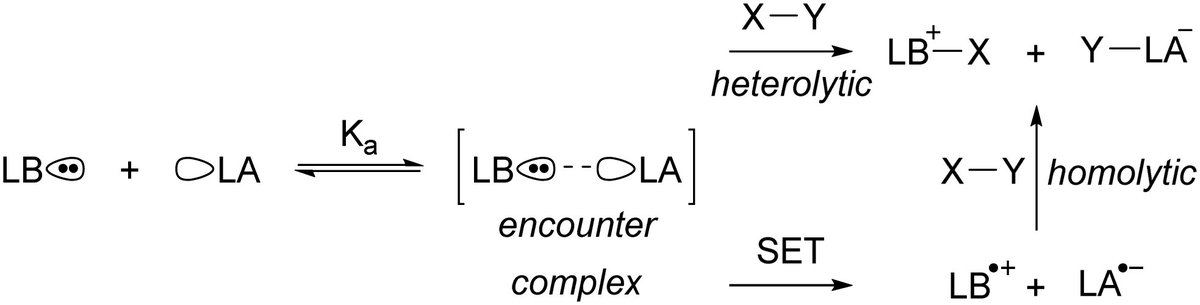
Mechanistic studies on single-electron transfer in frustrated Lewis pairs and its application to main-group chemistry (Chris Slootweg): pubs.rsc.org/en/content/art… (@ChemSocRev).

Tetramerization of BEB-Doped Phenalenyls to Obtain (BE)8-[16]Annulenes (E = N, O) (WagnerLab, Alexander Scholz, Dr Cate S. Anstöter): pubs.acs.org/doi/full/10.10… (@J_A_C_S).
![Boron-Chem-Research (@Boron_Chemistry) on Twitter photo 2024-04-19 18:20:08 Tetramerization of BEB-Doped Phenalenyls to Obtain (BE)8-[16]Annulenes (E = N, O) (@MWagnerLab, @AlexScholzinger, @CAnstoter): pubs.acs.org/doi/full/10.10… (@J_A_C_S). Tetramerization of BEB-Doped Phenalenyls to Obtain (BE)8-[16]Annulenes (E = N, O) (@MWagnerLab, @AlexScholzinger, @CAnstoter): pubs.acs.org/doi/full/10.10… (@J_A_C_S).](https://pbs.twimg.com/media/GLjCTq7XcAEpucR.jpg)

Effect of the U-Shaped Cavity of Conformationally Flexible Chiral Lewis-Acidic Boron-Based Catalysts in Multiselective Diels–Alder Reactions (J Org Chem/Org Lett): pubs.acs.org/doi/10.1021/ac… (@PIodide).



Reversible [4 + 1] Cycloaddition of Arenes by a “Naked” Acyclic Aluminyl Compound (J. Am. Chem. Soc.): pubs.acs.org/doi/10.1021/ja… (@DebotraSarkar, Aldridge Group).
![Boron-Chem-Research (@Boron_Chemistry) on Twitter photo 2024-04-18 19:39:37 Reversible [4 + 1] Cycloaddition of Arenes by a “Naked” Acyclic Aluminyl Compound (@J_A_C_S): pubs.acs.org/doi/10.1021/ja… (@DebotraSarkar, @GroupAldridge). Reversible [4 + 1] Cycloaddition of Arenes by a “Naked” Acyclic Aluminyl Compound (@J_A_C_S): pubs.acs.org/doi/10.1021/ja… (@DebotraSarkar, @GroupAldridge).](https://pbs.twimg.com/media/GLeKpT0XQAEYnOK.jpg)



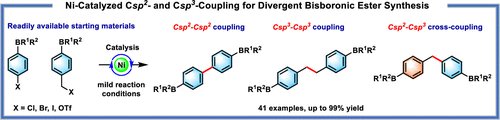
Ni-Catalyzed Csp2 and Csp3 Coupling for Divergent Bisboronic Ester Synthesis (J Org Chem/Org Lett): pubs.acs.org/doi/10.1021/ac….

Carborane-Arene Fused Boracyclic Analogues of Polycyclic Aromatic Hydrocarbons Accessed by Intramolecular Borylation (Chemical Science): pubs.rsc.org/en/content/art… (@MartinGroupBU, Yijie Li, Dr. M. Tamil, Manjur Oyasim Akram (মঞ্জুর ওয়াসিম আক্রাম)).


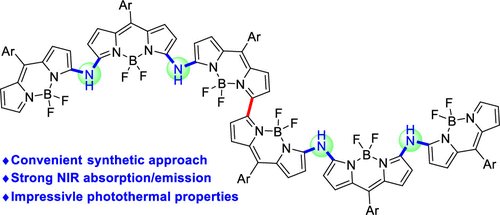
ucleophilic Aromatic Substitution (SNAr) as an Approach to Challenging Nitrogen-Bridged BODIPY Oligomers (J Org Chem/Org Lett): pubs.acs.org/doi/10.1021/ac….


We are hiring ! #PhD position starting in Oct 2024 in Organic Chemistry.
Offer below👇 Repost appreciated🙏!
CNRS Chimie Organic Chemistry Division from the SCF Aix-Marseille Université