Zhang Boyi
@zhangboyi9
PhD candidate in Prof. Wu Jishan's group🇸🇬
ID: 1354602260370575362
28-01-2021 01:28:50
58 Tweet
89 Followers
74 Following

This piece of work is now officially published in Nature Synthesis. Thank all reviewers for their constructive comments! nature.com/articles/s4416…

Aromaticity in 2D fully conjugated multicyclic macrocycles! We demonstrate nearly "one-pot" synthesis and show how the macrocycles interplay with each other at different redox states. Paper was just published in J. Am. Chem. Soc. pubs.acs.org/doi/10.1021/ja…


Delighted and excited to share the first publication from my group at Zhejiang University out in J. Am. Chem. Soc.! We report the topochemical synthesis of soluble and processable polymeric single crystals with rigid polycationic backbones. Many thanks to Fraser Stoddart bit.ly/3J807pI

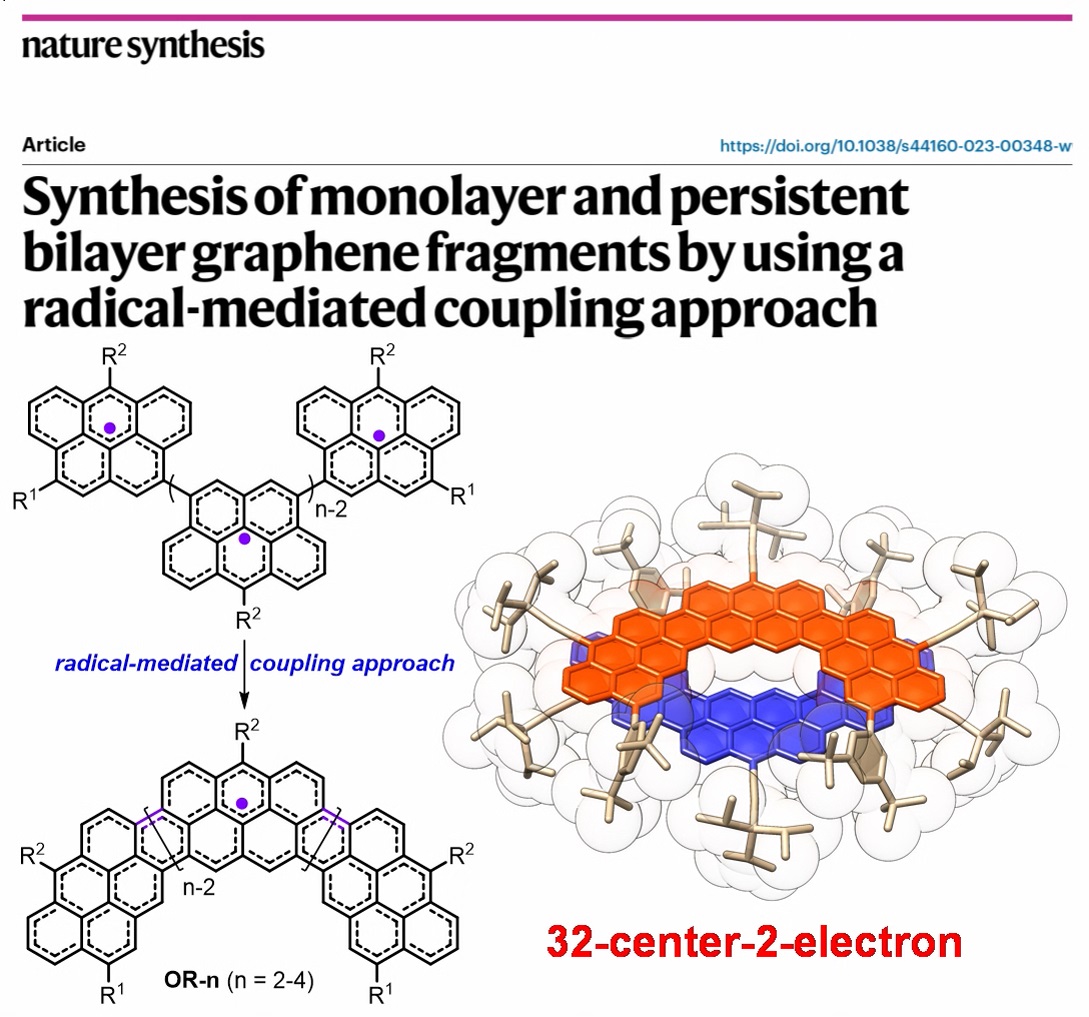
Radical-radical coupling and radical-mediated coupling approaches toward monolayer and bilayer graphene fragments! We also observed an unusual 32-center-2-electron covalent π-bonding. Paper was just published in Nature Synthesis. Tianyu Jiao SunZhe nature.com/articles/s4416…

Extended zethrenes showing a stable helical structure with an end-to-end twist of up to 201 dgree! Paper was just published in Chemical Science as part of the special issue to celebrate the 130th anniversary of Wuhan University. Sun Zhitao SunZhe pubs.rsc.org/en/content/art…


Circumpentacene has been synthesized and exhibits open-shell diradical charater, good stability, and amphoteric redox behavior. Find out more in our new Angewandte Chemie research article onlinelibrary.wiley.com/doi/10.1002/an… Congrats to Qing, Haipeng and Xudong Hou. NUS Chemistry @ResearchFos




Together with Harry Anderson Group we made an antiaromatic carbon allotrope. It is a molecule called cyclo[16]carbon. And it even is doubly-antiaromatic. What that means and how that was done, you can read in Nature: nature.com/articles/s4158…


A core expanded [10]annulene! We observed co-existence of Möbius/Hückel topology and aromaticity/antiaromaticity in its dication, presumably due to the balance between strain and aromaticity. Paper was just published Angewandte Chemie : onlinelibrary.wiley.com/doi/10.1002/an…
![Jishan Wu (@jishan_wu) on Twitter photo A core expanded [10]annulene! We observed co-existence of Möbius/Hückel topology and aromaticity/antiaromaticity in its dication, presumably due to the balance between strain and aromaticity. Paper was just published <a href="/angew_chem/">Angewandte Chemie</a> : onlinelibrary.wiley.com/doi/10.1002/an… A core expanded [10]annulene! We observed co-existence of Möbius/Hückel topology and aromaticity/antiaromaticity in its dication, presumably due to the balance between strain and aromaticity. Paper was just published <a href="/angew_chem/">Angewandte Chemie</a> : onlinelibrary.wiley.com/doi/10.1002/an…](https://pbs.twimg.com/media/GERfOkWbUAA-BRs.jpg)




π-Extended 9b-Boraphenalenes: Synthesis, Structure, and Physical Properties (J. Am. Chem. Soc.): pubs.acs.org/doi/10.1021/ja….


Aza-Superbenzene and Aza-Supernaphthalene: Dications, tetracations, and hexacations of a tetraazasuperbenzene and a hexaazasupernaphthalene show global aromaticity or anti-aromaticity (work published in Angewandte Chemie) chemistryviews.org/aza-superbenze…


A rhombus-shaped hydrocarbon, [4]rhombene, was synthesized and applied for organic lasers with emission beyond 830 nm. This collaborative work with Maria's team is now published in Angewandte Chemie. Congratulations to Shen Tong and other co-authors! onlinelibrary.wiley.com/doi/10.1002/an…
![Jishan Wu (@jishan_wu) on Twitter photo A rhombus-shaped hydrocarbon, [4]rhombene, was synthesized and applied for organic lasers with emission beyond 830 nm. This collaborative work with Maria's team is now published in <a href="/angew_chem/">Angewandte Chemie</a>. Congratulations to <a href="/ShenTon44744598/">Shen Tong</a> and other co-authors! onlinelibrary.wiley.com/doi/10.1002/an… A rhombus-shaped hydrocarbon, [4]rhombene, was synthesized and applied for organic lasers with emission beyond 830 nm. This collaborative work with Maria's team is now published in <a href="/angew_chem/">Angewandte Chemie</a>. Congratulations to <a href="/ShenTon44744598/">Shen Tong</a> and other co-authors! onlinelibrary.wiley.com/doi/10.1002/an…](https://pbs.twimg.com/media/GSFyXEXbgAEPyIg.jpg)