
Max Crüsemann
@mcrusemann
Natural products researcher, interested in biosynthesis, genome mining, discovery and metabolomics
ID: 1276077413999534080
http://pharma.uni-bonn.de/pharmazeutische-biologie/en/workgroups/wg-crusemann/ 25-06-2020 08:59:09
671 Tweet
1,1K Followers
536 Following

Activation of Primary C–H Bonds in Oxidative Cyclizations of Tambjamines Catalyzed by Rieske Oxygenases TamC and PtTamC | Journal of the American Chemical Society Queen's University Queenschem Kingston Ross Group @ Queen’s pubs.acs.org/doi/10.1021/ja…

Ogasawara (A03) and Ushimaru (A01) groups, in collaboration with Prof. Chang in North Carolina State University, revealed the importance of stereoelectronic effect in nonheme Iron enzyme catalyzing cyclopropanation. J. Am. Chem. Soc. doi.org/10.1021/jacs.4…

Single-Enzyme Conversion of Tryptophan to Skatole and Cyanide Expands the Mechanistic Competence of Diiron Oxidases | Journal of the American Chemical Society Brandeis University UC San Diego Scripps Institution of Oceanography UC San Diego Skaggs School of Pharmacy pubs.acs.org/doi/10.1021/ja…

Out today in Angewandte Chemie Evolution-Guided Discovery of Antimycobacterial Triculamin-Like Lasso Peptides doi.org/10.1002/anie.2… Fun collaboration with the Tørring lab Thomas Tørring

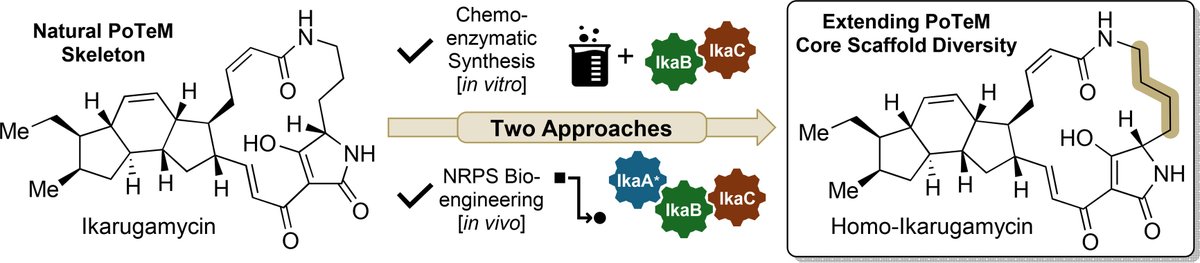
Expanding Polycyclic Tetramate Macrolactam (PoTeM) Core Structure Diversity by Chemo-Enzymatic Synthesis and Bioengineering (Tobias A. M. Gulder and co-workers) Manuel Einsiedler Sabine Schneider TAMGulderLab_TechBioDD Antibiotikaforschung (inaktiv) Helmholtz-Zentrum für Infektionsforschung –inaktiv #openaccess 🔓onlinelibrary.wiley.com/doi/10.1002/an…



A beauty put together by our Tobias Milzarek Antibiotikaforschung (inaktiv) Helmholtz-Zentrum für Infektionsforschung –inaktiv. It summarizes a huge body of work by many on one of our favorite natural product classes, the sorbicillinoids! Thanks to DFG public | @[email protected] for funding!

The Kuzuyama group (A01), in collaboration with Prof. Jeroen S. Dickschat, has identified a pair of cyanobacterial terpene synthases to produce a rare 6,6,7-tricyclic diterpene alcohol, deepening our mechanistic understanding of terpene cyclases.J. Am. Chem. Soc. pubs.acs.org/doi/full/10.10…
Lab article: We revealed the significance of the cryptic amino group of the quinone intermediate common to the biosynthesis of tetrahydroxynaphthalene-derived meroterpenoids such as furaquinocin/naphterpin. Chemical Science pubs.rsc.org/en/content/art…

excited to see our paper on the total synthesis and biosynthetic logic of Lugdunomycin out in Journal of the American College of Surgeons (JACS). Congrats to Michiel Uiterweerd and Isabel Nuñez. Awesome collaboration with the Minnaard lab. pubs.acs.org/doi/10.1021/ja…


Our work on Taxol biosynthesis is now out at Nature Synthesis. You can read it here: nature.com/articles/s4416… Congratulations to Feiyan Liang and the rest of the Kampranis Lab and thank you Novo Nordisk Foundation DK Frie Forsk.fond and Department of Plant & Environmental Sciences




Thrilled to share our latest paper just out in J. Am. Chem. Soc.! We uncovered an unprecedented KS domain-catalyzed β-lactonization in the biosynthesis of the HMG-CoA synthase inhibitor hymeglusin. Huge congratulations to Mizuki on his first paper! 🎉 pubs.acs.org/doi/10.1021/ja…

Super happy that EnzyMS, our LC-MS pipeline for detecting promiscuous #enzyme activity, is out in Chem Catalysis ! Testing it on WelO5* + soraphen A uncovered an oxidative demethylation - missed by standard tools. Curious? 👉 cell.com/chem-catalysis…



