Mackenzie Thompson
@mackthompson88
Post doctoral researcher at the University of California, Berkeley.
ID: 1240977836665647106
20-03-2020 12:25:54
83 Tweet
60 Followers
128 Following
Proud to share our latest work in Biophysical Journal authors.elsevier.com/a/1gW271SPT41la Nanodiscs are an exciting technology for studying membrane proteins in lipid bilayers. We asked whether nanodisc bilayers preserve the native properties of intact lipid membranes. And the answer is… NO! 1/2

Great to see our story led by David Kern on LRRC8A:C volume regulated anion channels in its final form! Lots of new data since the preprint including structures of a mutant that disrupts lipid block of the pore to activate the channel rdcu.be/c7MWW nature.com/articles/s4159…

Check out our latest work published in Nature Communications. Congrats to Christian Tessier , Johnathon Emlaw, and Mich for all the hard work. nature.com/articles/s4146…


I am excited to share that the paper from my Ph.D. work in the Hibbs lab is out in Nature Communications today. It was a great experience working on this project with @RyanHibbs10, Mahfuz Rahman, and Jinfeng Teng. nature.com/articles/s4146… UTSW Graduate School of Biomedical Sciences The Department of Biophysics at UT Southwestern



Kudos to John Baenziger & his uOttawa | Faculté de médecine, Faculty of Medicine team for more impactful insights into a key receptor that is implicated in neurological and neuromuscular diseases including #Alzheimer’s & #Parkinson’s. 🙌





Collaborative effort by John Baenziger, Hugues Nury, and daCostaLab. Congrats to Mackenzie Thompson for leading the cryo-EM work and Christian Tessier for leading the single-channel recordings. Happy to have contributed.


Congrats to Mackenzie Thompson for the crowning achievement of his PhD. Thanks to my outstanding collaborators: Eleftherios Zarkadas, Hugues Nury , daCostaLab , François Dehez, and the many outstanding trainee contributors: Christian Tessier, Anna Ananchenko, and Johnathon Emlaw

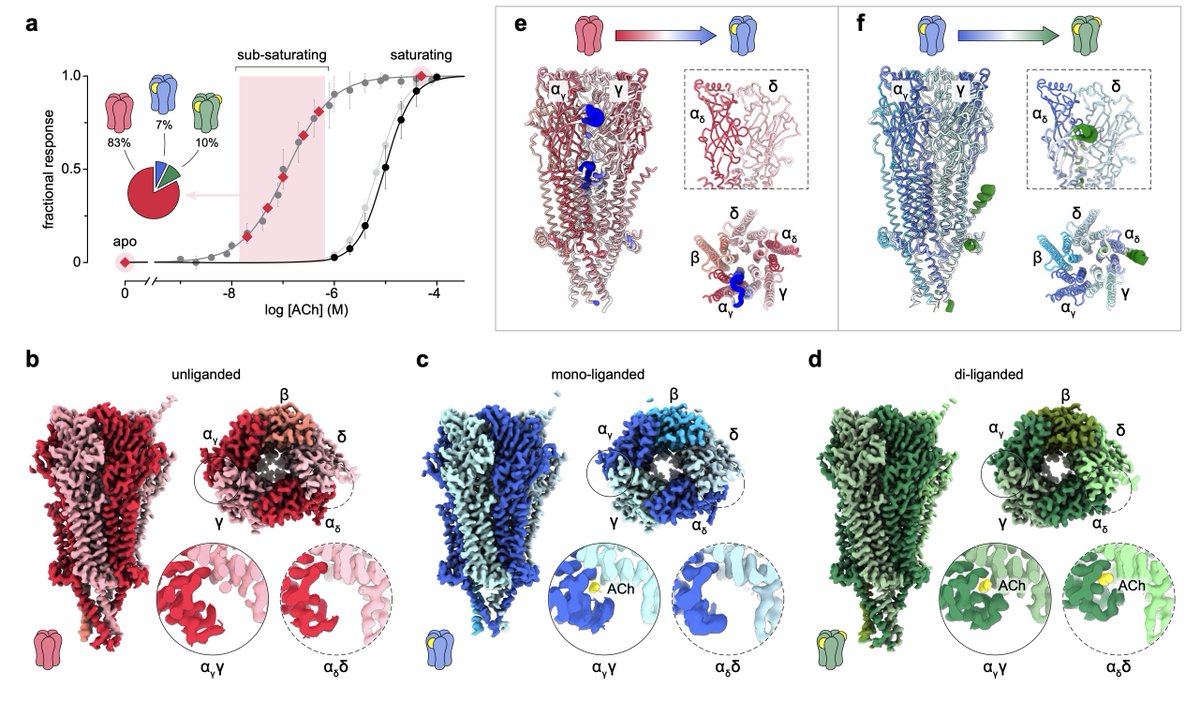
A new publication from John Baenziger lab reveals a sequential activation mechanism for the Torpedo nAChR! These new structures show how #nAChR subunits transition between unliganded to mono- and di-liganded conformations upon agonist binding.