
Josef Boronski
@josefboronski
Organometallic Chemistry | Metal-Metal Bonding | Lecturer @impchemistry |orcid.org/0000-0002-1435… |
ID: 1315964058835849217
https://profiles.imperial.ac.uk/j.boronski 13-10-2020 10:35:09
403 Tweet
852 Takipçi
493 Takip Edilen

Our latest Al-Fe research is published in J. Am. Chem. Soc.. Led by my student Roushan Chemistry at UIC and our collaboration with Dan Ess of BYU, we are starting to map the chemistry of Al(II) through this mild approach! doi.org/10.1021/jacs.5…

Years in the making – 3-centre-1-electron trithorium metal-metal bonding. Out now in Nature Chemistry ! nature.com/articles/s4155…


We still have a PhD position open in our team Warwick Chemistry The deadline is 29th April, and funding is secured for 3.5 years (UK applicants only). Please email me with any queries. RTs are appreciated! 🙏🏼🙏🏼

We discovered an in crystallo organometallic isomerization that shows 4f-orbitals can (uniquely) promote chemical reactions among isostructural 3d, 4d, 5d, 4f, & 5f complexes! Check out our latest paper, out now Nature Chemistry rdcu.be/eiVZw


New paper out today in Nature Synthesis from Wenbang Yang (Wenbang Yang). We describe addition of Zn–Zn bonds to main group carbenes. The system nicely illustrates the continuum between oxidative addition and reductive addition processes. rdcu.be/ejPSS


Exciting new ChemRxiv preprint on sustainable fluorine chemistry. Dr. Niko A. Jenek, Sarah Patrick, Andreas Phanopoulos and Jiahuang Mao report a simple one-pot transfer fluorination method to recycle fluoride content of HFCs, HFOs, fluoroethers, and PFAS. chemrxiv.org/engage/chemrxi…


Christoph's paper on an exptal and computational study of the 1st cmpds with Be-N and Be-O triple bond character is now out in J. Am. Chem. Soc.. Great (and essential) 1st collab. outing with David J. D. Wilson La Trobe Institute for Molecular Science. #ozchem Humboldt-Stiftung AFOSR Monash Chemistry tinyurl.com/7hmzzzcz



Do you take tea breaks with your colleagues? Discoverer of the electron J. J. Thomson was a firm believer in taking tea breaks. As the leader of the research students at Cavendish laboratory at Cambridge University, Thomson established a daily tea break with students.



Breaking bonds at tin(II)! We've had a look at the spectrum of bond addition reactions, from reductive (Be-Be) to oxidative (Cl-Cl), and everything in between (B-B, B-H, H-H) Angewandte Chemie Aldridge Group Maximilian Dietz Amelia Swarbrook doi.org/10.1002/anie.2…


Jack, Aaron, and Maya present an online angular overlap calculator, which can calculate d-orbital splitting for any geometry. Play with it here: aom.hunter-lab.com. Student and teacher manuals in J. Chem. Ed. pubs.acs.org/doi/full/10.10… ACS Publications Northwestern Chemistry

Matt (Matthew Evans) and co's work on the first main group metal-P4 complexes, redn of P4 to [P4]2- with a Mg-N2 complex, and hydrolysis of [P4]2- to PH3, has been accepted Chem. tinyurl.com/bdewbze2 Free access: tinyurl.com/23vbtuju #ozchem Monash Science
![Jones Research Group (@jones_research) on Twitter photo Matt (<a href="/EvansInorganic/">Matthew Evans</a>) and co's work on the first main group metal-P4 complexes, redn of P4 to [P4]2- with a Mg-N2 complex, and hydrolysis of [P4]2- to PH3, has been accepted <a href="/Chem_CP/">Chem</a>. tinyurl.com/bdewbze2 Free access: tinyurl.com/23vbtuju
#ozchem <a href="/Monash_Science/">Monash Science</a> Matt (<a href="/EvansInorganic/">Matthew Evans</a>) and co's work on the first main group metal-P4 complexes, redn of P4 to [P4]2- with a Mg-N2 complex, and hydrolysis of [P4]2- to PH3, has been accepted <a href="/Chem_CP/">Chem</a>. tinyurl.com/bdewbze2 Free access: tinyurl.com/23vbtuju
#ozchem <a href="/Monash_Science/">Monash Science</a>](https://pbs.twimg.com/media/Gu9u5llb0AMazop.jpg)

🔥Excited to share our latest: "C(sp2)–H Bond Activation with a Heterometallic Nickel---Aluminium Complex” Now out in Angewandte Chemie w/ Crimmin Group Work done by PhD student, Joseph A. Zurakowski w/ Benedek Stadler & Mark Crimmin @ ICL! 💪 tinyurl.com/3bbj56nw

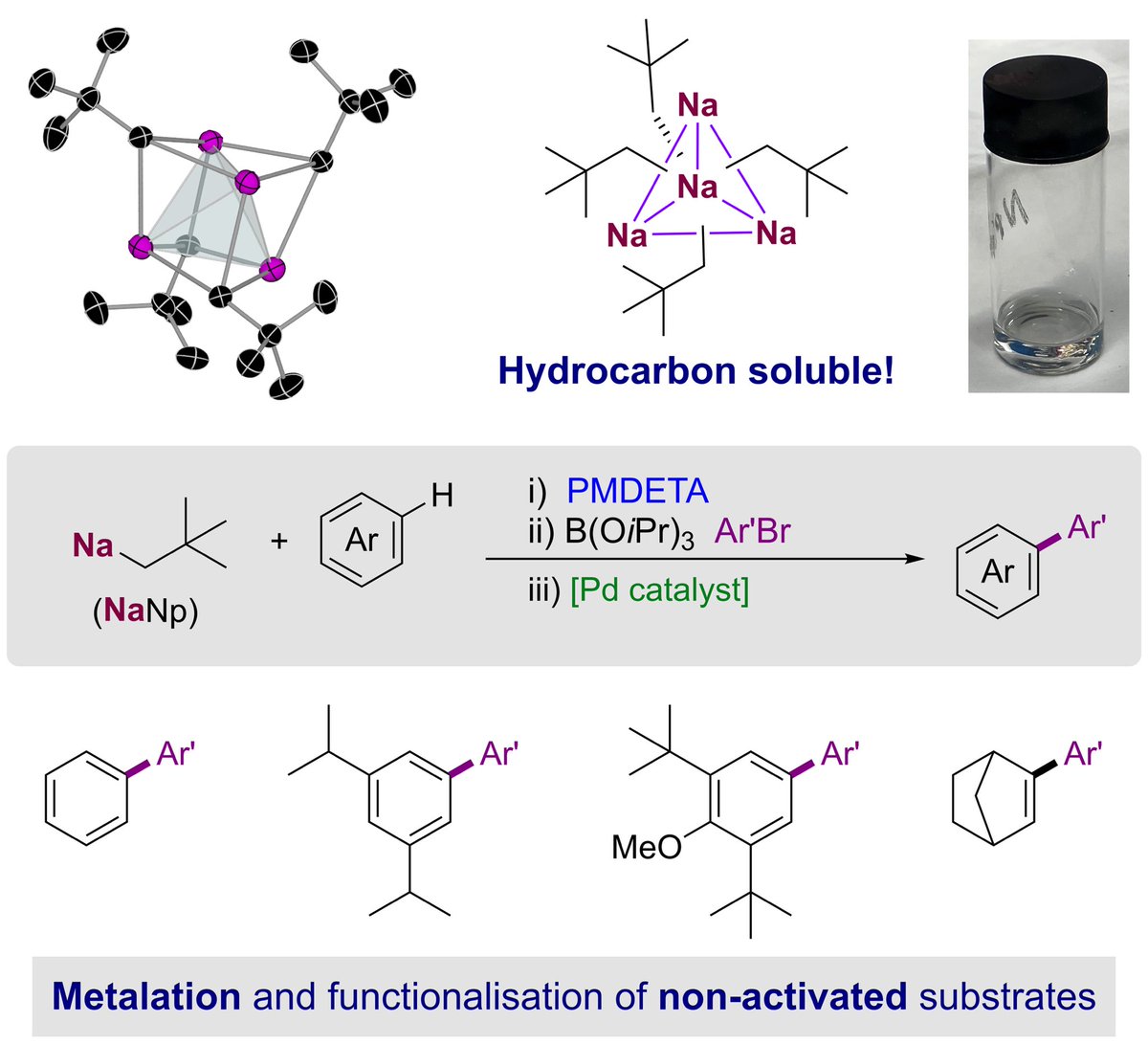
Excited to share David Anderson latest work, just accepted Angewandte Chemie on the structure and applications of #sodium #neopentyl a donor free #organosodium reagent soluble in #hydrocarbon solvents DCBPunibern #OpenAccess onlinelibrary.wiley.com/doi/epdf/10.10…

Excited to see this work published today in J. Am. Chem. Soc. , fun chemistry and a real group effort here by Dr Claire McMullin , Agustín Morales, Mary Mahon and big Mike! (Chemistry@Bath Uni) pubs.acs.org/doi/10.1021/ja…

Our groups first foray into heterobimetallic Zn/Al chemistry. Congrats to Jake, Josef, Avantika, George, Nik and MillsGroup chemrxiv.org/engage/chemrxi…
