Gui Lab
@jinghan_gui
ID: 1005006689584599040
http://guigroup.sioc.ac.cn 08-06-2018 08:40:41
89 Tweet
803 Followers
242 Following


Check out our recent syntheses of bufospirostenin A and ophiopogonol A in only seven steps from inexpensive starting materials just out in J. Am. Chem. Soc. . Great collaboration with Hong group from Zhejiang University for computational studies: pubs.acs.org/doi/10.1021/ja…




Modern alchemy appearing today in Science Magazine : science.org/doi/10.1126/sc… Commentary (thank you Prof. Ye!): science.org/doi/10.1126/sc…

Our 13-step synthesis of the rearranged steroid phomarol, which features a biomimetic, stereospecific SN2’ cyclization and a convergent fragment-coupling strategy, is now out in J. Am. Chem. Soc. : pubs.acs.org/doi/10.1021/ja…


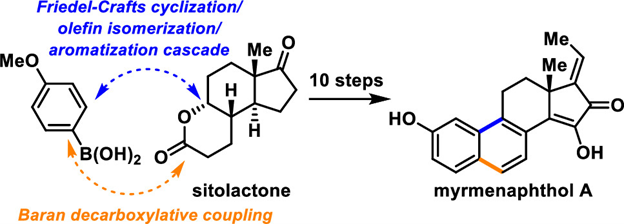
Check out our latest review on the efficient syntheses of steroid, terpenoid and alkaloid natural products by a skeletal reorganization strategy, out now in Nat. Prod. Reports : rsc.li/3UpPp4s
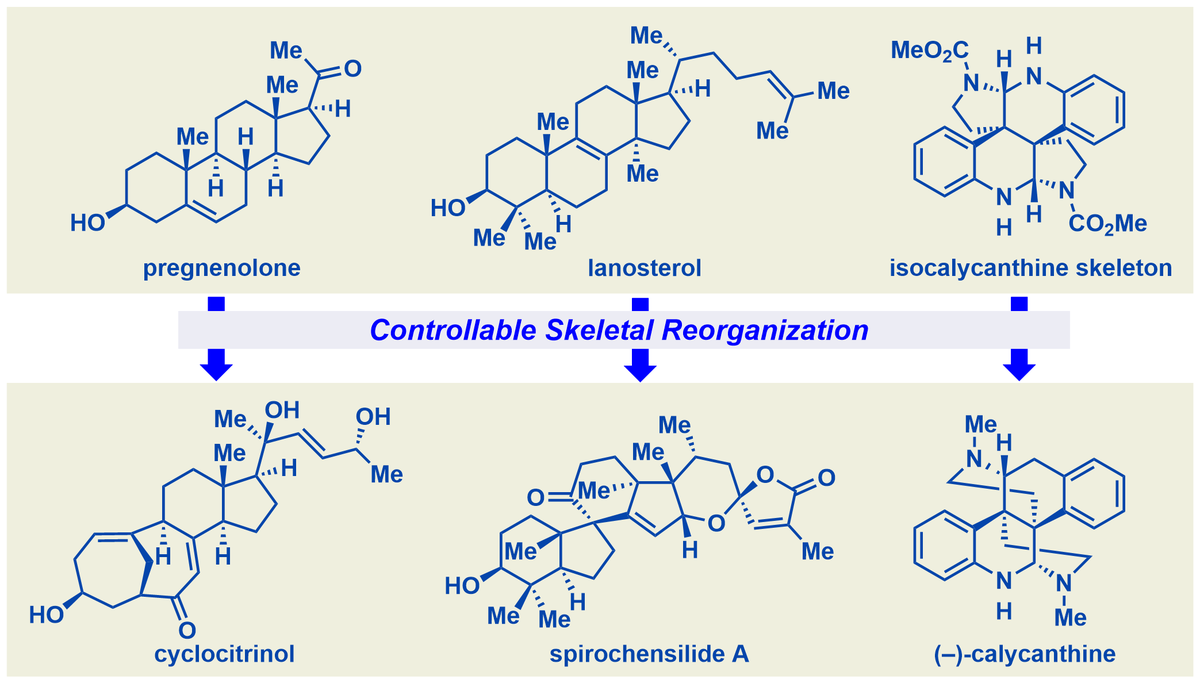

Our first work on the chemoenzymatic synthesis: using KSH enzyme as the biocatalyst enables the steroidal 9α-hydroxylation and B-ring fragmentation cascade to give 9,10-secosteroids. Great collaboration with Prof. Wen Liu at SIOC. Out now in Angewandte Chemie : bit.ly/3UHm5q8

Our latest synthesis of the 18,22-cyclosterols aspersteroids A and B, which features an interesting radical relay cyclization and a Ti(III)-mediated diastereoselective epoxide reduction, is now out in J. Am. Chem. Soc.: bit.ly/3IiAYYv

Published today in Science Magazine : science.org/doi/epdf/10.11…

The final version of this work was published in Nature Chemistry : nature.com/articles/s4155…


Our synthesis of the bisnortriterpenoid rubriflordilactone B, which features a [2,3]-Wittig−Still rearrangement, a Friedel−Crafts cyclization and an E1cB reaction/transesterification/oxa-Michael addition cascade, is now out in J. Am. Chem. Soc. : bit.ly/430IyCx

Our recent work in steroid synthesis, which features a tandem Negishi/Heck cross-coupling and a Baran reductive olefin coupling (BROC), is now out in J. Am. Chem. Soc.: pubs.acs.org/doi/10.1021/ja…


Our syntheses of harziane diterpenoids (including harziandione and harzianone) are now out in J. Am. Chem. Soc. . This work highlights again the power of Baran reductive olefin coupling (BROC) in building sterically hindered C−C bonds: pubs.acs.org/doi/10.1021/ja…