
kematsuda
@kematsuda2
Kenichi Matsuda, Natural products, Biosynthesis, Biocatalysis, Genome-mining, Cyclic peptides, Associate Prof. @ Hokkaido University, Japan
ID: 1244771569659871232
https://scholar.google.co.jp/citations?user=fTbHr-oAAAAJ&hl=ja 30-03-2020 23:40:56
157 Tweet
313 Takipçi
328 Takip Edilen

Join us! We are looking for a new team member (PhD student) with strong background in organic chemistry. 🙏 RETWEET (We want to recruit internationally) Organic chemistry meets #DirectedEvolution Highly interdisciplinary & passionate research group uni-bielefeld.hr4you.org/job/view/4339/…

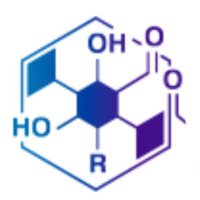
Thrilled to share our latest paper just out in J. Am. Chem. Soc.! We uncovered an unprecedented KS domain-catalyzed β-lactonization in the biosynthesis of the HMG-CoA synthase inhibitor hymeglusin. Huge congratulations to Mizuki on his first paper! 🎉 pubs.acs.org/doi/10.1021/ja…





The Ushimaru group (A01), in collaboration with Prof. Hung-wen Liu, has uncovered a sulfur incorporation mechanism generating the thionucleoside antibiotic albomycin. nature.com/articles/s4192… Nature Catalysis



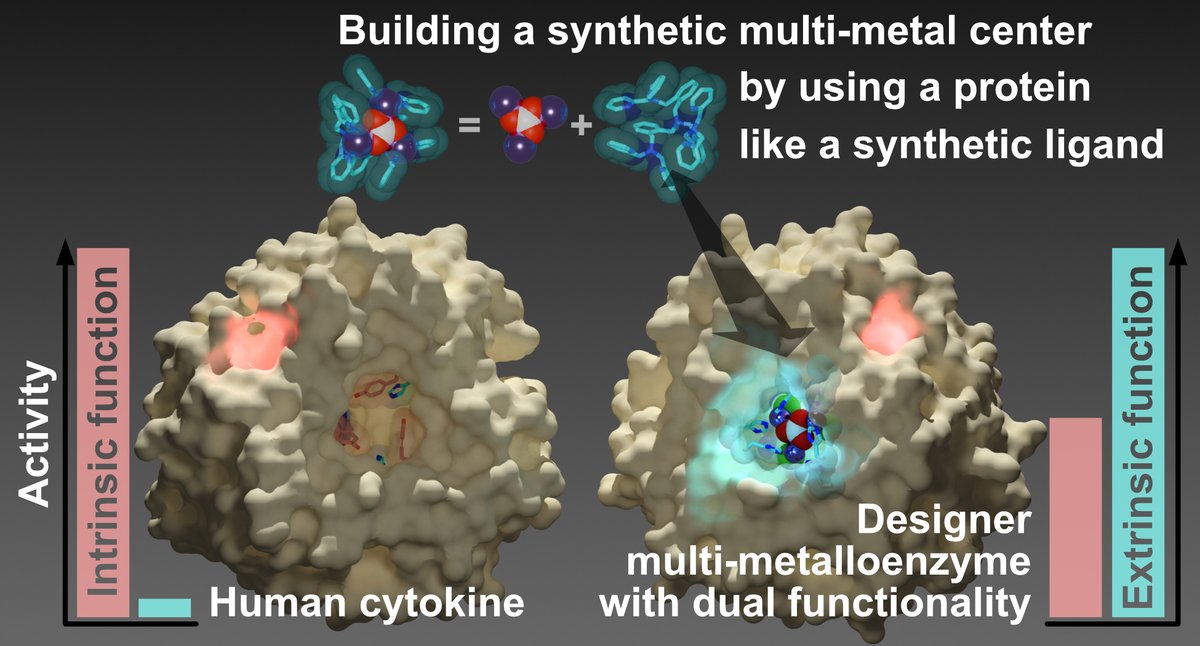
渾身の新作論文Nature Communications (doi.org/10.1038/s41467…)です!合成多核金属錯体の構造をタンパク質だけを用いて構築に成功しました。また、人工酵素の多くがテンプレートタンパク質元来の機能と人工機能をトレードオフする中、今回の人工酵素では、内在性機能と外来性機能を両立しています。(1/6)


The Kato (A03) and Shoji (A03) groups developed an efficient system to express active hemoproteins by engineering the heme biosynthesis pathway in E. coli, improving whole-cell and lysate-based biocatalysis using heme enzymes. Angewandte Chemie onlinelibrary.wiley.com/doi/10.1002/an…



The Tsutsumi group (A02) established a strategy combining comparative genomics and metabolomics analyses to rapidly discover natural products, leading to the identification of novel tricyclic peptides lentindoles A & B from Lentzea sp. OK19-0192. ChemEurJ: Chemistry - A European Journal …mistry-europe.onlinelibrary.wiley.com/doi/10.1002/ch…




