The Hari Group
@harigroupiisc
Organic Synthesis Research Group @iiscbangalore
ID: 1393134424498196482
http://theharigroup.in 14-05-2021 09:21:56
1,1K Tweet
2,2K Followers
446 Following



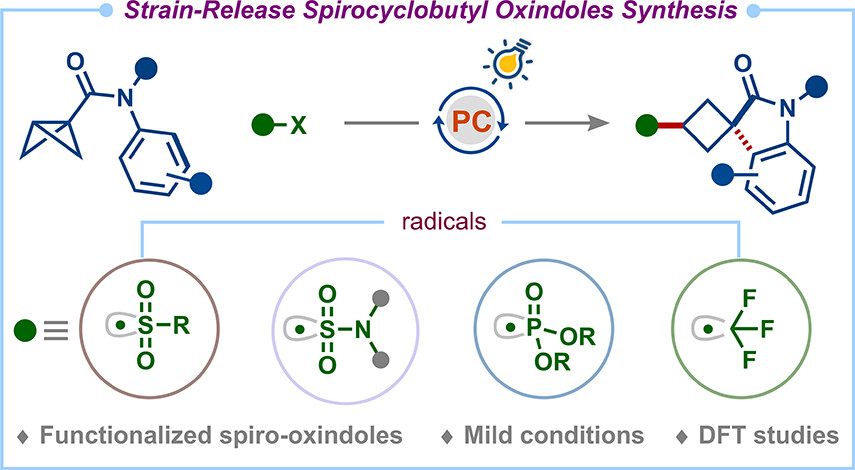
🚀Our work on Strain-release radical cascade for the synthesis of spirocyclobutyl oxindoles, previously on ChemRxiv , is now published in Organic Letters J Org Chem/Org Lett . Thanks, SERB IISc Bangalore, for the funding. 🔗pubs.acs.org/doi/10.1021/ac…

Our catalytic, strain-enabled strategy for accessing functionalized skipped dienes in a highly stereoselective fashion is now out in ACS Catalysis. Thanks, SERB IISc Bangalore, for funding. Thanks, CCL@IISc, for the DFT studies. pubs.acs.org/doi/full/10.10…




A research account of our work on strain-enabled spirocyclization cascades has been published SYNLETT Journal. Thank you, IISc Bangalore SERB, for the financial support. thieme-connect.com/products/ejour…




Thank you, Thieme India, for this fantastic opportunity to discuss our research findings.

Deconstruction of Cyclic Ketones: Our work on the electrochemical interrupted Dowd–Beckwith reaction for deconstructive functionalization of cyclic ketones is out in Angewandte Chemie. Thanks to IISc Bangalore and Anusandhan National Research Foundation for the financial support. onlinelibrary.wiley.com/doi/abs/10.100…
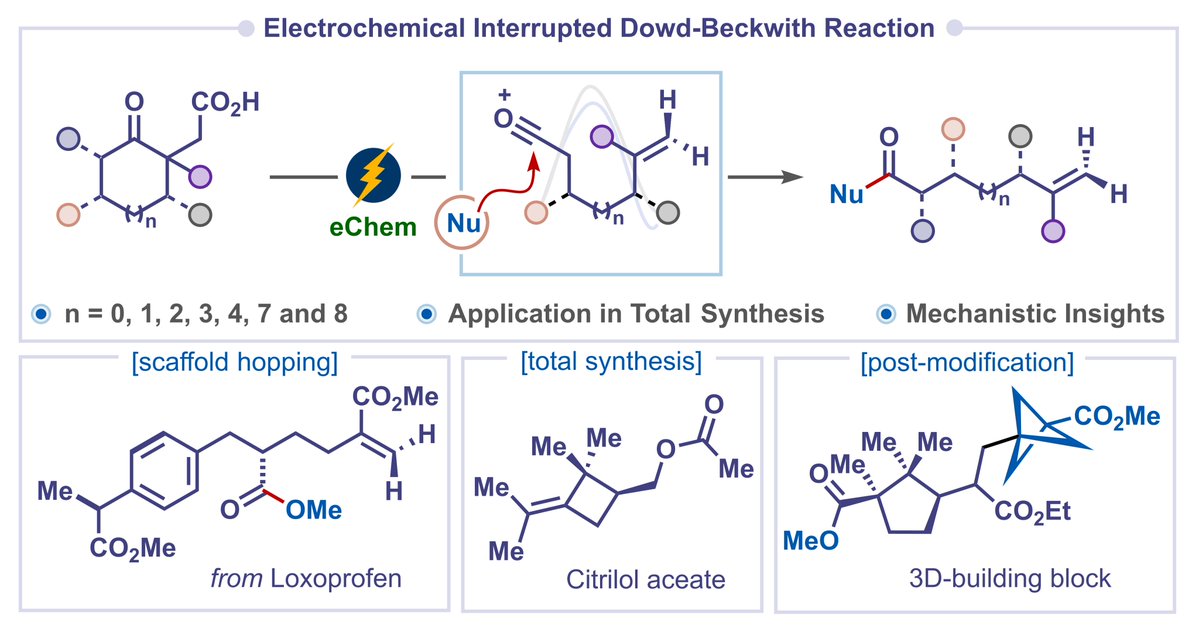

Deconstructive Functionalization of Cyclic Ketones via Electrochemical Interrupted Dowd–Beckwith Reaction (Durga Prasad Hari and co-workers) The Hari Group • onlinelibrary.wiley.com/doi/10.1002/an…
Burgenstock #bc_scs25 Editorial Board meeting Rebecca Buller AlbrechtResearch Olalla Vázquez KatayevLab The Hari Group R. Mulvey Prof Hevia 🇺🇦 Lacour Lab Waser Group L. De Luca Sascha Hoogendoorn Michal Juricek LIAC at EPFL F. Gallou and Gasser Group


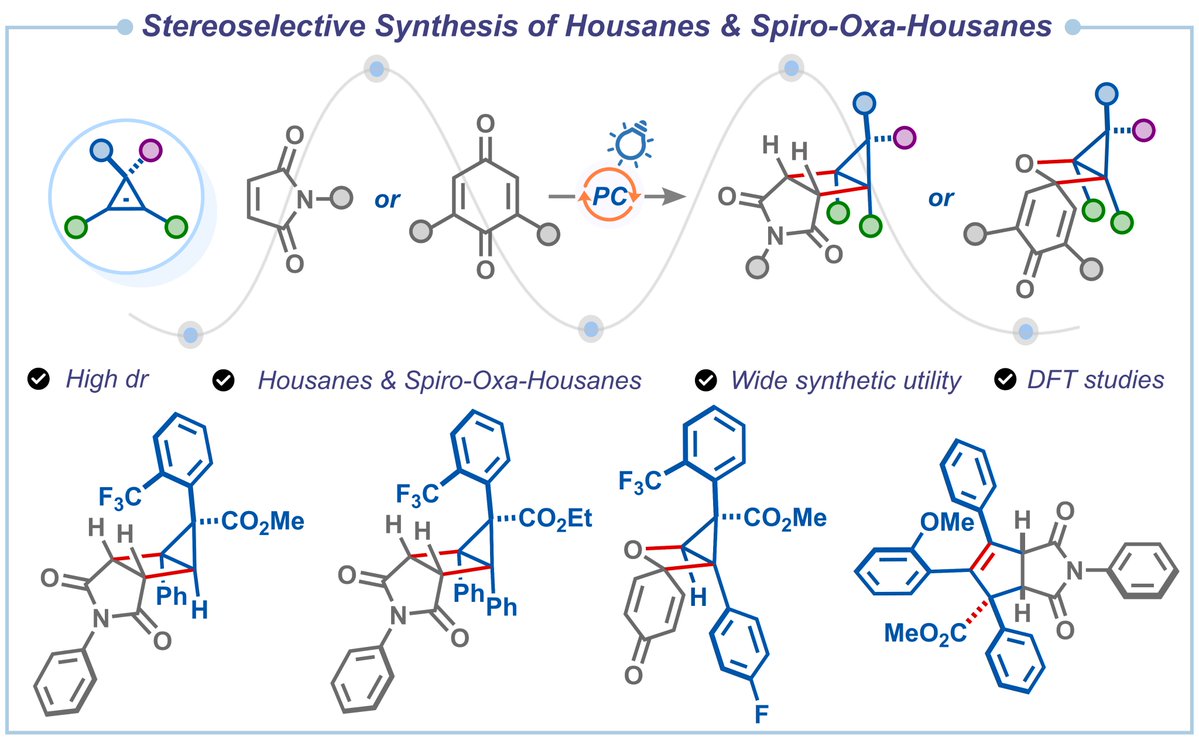
🏠Housanes: Our work on the stereoselective synthesis of (oxa)housanes and their skeletal remodeling to access bicyclic imides is out in Chemical Science. Thank you, Anusandhan National Research Foundation IISc Bangalore, for the funding. pubs.rsc.org/en/content/art…

🦋RPC@BCB: Our work on strain-enabled radical-polar crossover annulation to access hetero-bicyclo[3.1.1]heptanes just out in Angewandte Chemie. Thanks to IISc Bangalore and Anusandhan National Research Foundation for the financial support. onlinelibrary.wiley.com/doi/10.1002/an…
![The Hari Group (@harigroupiisc) on Twitter photo 🦋RPC@BCB: Our work on strain-enabled radical-polar crossover annulation to access hetero-bicyclo[3.1.1]heptanes just out in <a href="/angew_chem/">Angewandte Chemie</a>. Thanks to <a href="/iiscbangalore/">IISc Bangalore</a> and <a href="/ANRFIndia/">Anusandhan National Research Foundation</a> for the financial support.
onlinelibrary.wiley.com/doi/10.1002/an… 🦋RPC@BCB: Our work on strain-enabled radical-polar crossover annulation to access hetero-bicyclo[3.1.1]heptanes just out in <a href="/angew_chem/">Angewandte Chemie</a>. Thanks to <a href="/iiscbangalore/">IISc Bangalore</a> and <a href="/ANRFIndia/">Anusandhan National Research Foundation</a> for the financial support.
onlinelibrary.wiley.com/doi/10.1002/an…](https://pbs.twimg.com/media/GtNe4jwbMAA98IP.jpg)

Strain-Enabled Radical-Polar Crossover Annulation to Access Spiro-, Fused-, and Enantioenriched-Aza/Oxa-Bicyclo[3.1.1]Heptanes (Durga Prasad Hari and co-workers) The Hari Group • onlinelibrary.wiley.com/doi/10.1002/an…

Thank you Angewandte Chemie for this opportunity.

🚀Propellane→MCB→BCH: Our work on the first sigmatropic rearrangement with [1.1.1]propellane for accessing functionalized MCBs and their application in the synthesis of BCHs is out Nature Communications. Thank you, Anusandhan National Research Foundation and IISc Bangalore, for the funding. nature.com/articles/s4146…
![The Hari Group (@harigroupiisc) on Twitter photo 🚀Propellane→MCB→BCH: Our work on the first sigmatropic rearrangement with [1.1.1]propellane for accessing functionalized MCBs and their application in the synthesis of BCHs is out <a href="/NatureComms/">Nature Communications</a>. Thank you, <a href="/ANRFIndia/">Anusandhan National Research Foundation</a> and <a href="/iiscbangalore/">IISc Bangalore</a>, for the funding.
nature.com/articles/s4146… 🚀Propellane→MCB→BCH: Our work on the first sigmatropic rearrangement with [1.1.1]propellane for accessing functionalized MCBs and their application in the synthesis of BCHs is out <a href="/NatureComms/">Nature Communications</a>. Thank you, <a href="/ANRFIndia/">Anusandhan National Research Foundation</a> and <a href="/iiscbangalore/">IISc Bangalore</a>, for the funding.
nature.com/articles/s4146…](https://pbs.twimg.com/media/GvQVPN7X0AA-d8L.jpg)

🚀Skeletal editing of ketones using [1.1.1]propellane to access functionalized MCB-ketones and spirocyclic scaffolds is out ChemRxiv. A fantastic collaboration with Mykhailiuk Chem 🇺🇦. Thank you, Anusandhan National Research Foundation and IISc Bangalore, for the financial support. chemrxiv.org/engage/chemrxi…
![The Hari Group (@harigroupiisc) on Twitter photo 🚀Skeletal editing of ketones using [1.1.1]propellane to access functionalized MCB-ketones and spirocyclic scaffolds is out <a href="/ChemRxiv/">ChemRxiv</a>. A fantastic collaboration with <a href="/MykhailiukChem/">Mykhailiuk Chem 🇺🇦</a>. Thank you, <a href="/ANRFIndia/">Anusandhan National Research Foundation</a> and <a href="/iiscbangalore/">IISc Bangalore</a>, for the financial support. chemrxiv.org/engage/chemrxi… 🚀Skeletal editing of ketones using [1.1.1]propellane to access functionalized MCB-ketones and spirocyclic scaffolds is out <a href="/ChemRxiv/">ChemRxiv</a>. A fantastic collaboration with <a href="/MykhailiukChem/">Mykhailiuk Chem 🇺🇦</a>. Thank you, <a href="/ANRFIndia/">Anusandhan National Research Foundation</a> and <a href="/iiscbangalore/">IISc Bangalore</a>, for the financial support. chemrxiv.org/engage/chemrxi…](https://pbs.twimg.com/media/GwNYFrKXwAAQhia.jpg)



![The Hari Group (@harigroupiisc) on Twitter photo Our work on the first sigmatropic rearrangement with [1.1.1]propellane for accessing functionalized MCBs and its application in the synthesis of BCHs is out <a href="/ChemRxiv/">ChemRxiv</a>. Thanks, <a href="/serbonline/">SERB</a> <a href="/iiscbangalore/">IISc Bangalore</a>, for the funding. doi.org/10.26434/chemr… Our work on the first sigmatropic rearrangement with [1.1.1]propellane for accessing functionalized MCBs and its application in the synthesis of BCHs is out <a href="/ChemRxiv/">ChemRxiv</a>. Thanks, <a href="/serbonline/">SERB</a> <a href="/iiscbangalore/">IISc Bangalore</a>, for the funding. doi.org/10.26434/chemr…](https://pbs.twimg.com/media/GapiYSmaMAAWVpC.jpg)
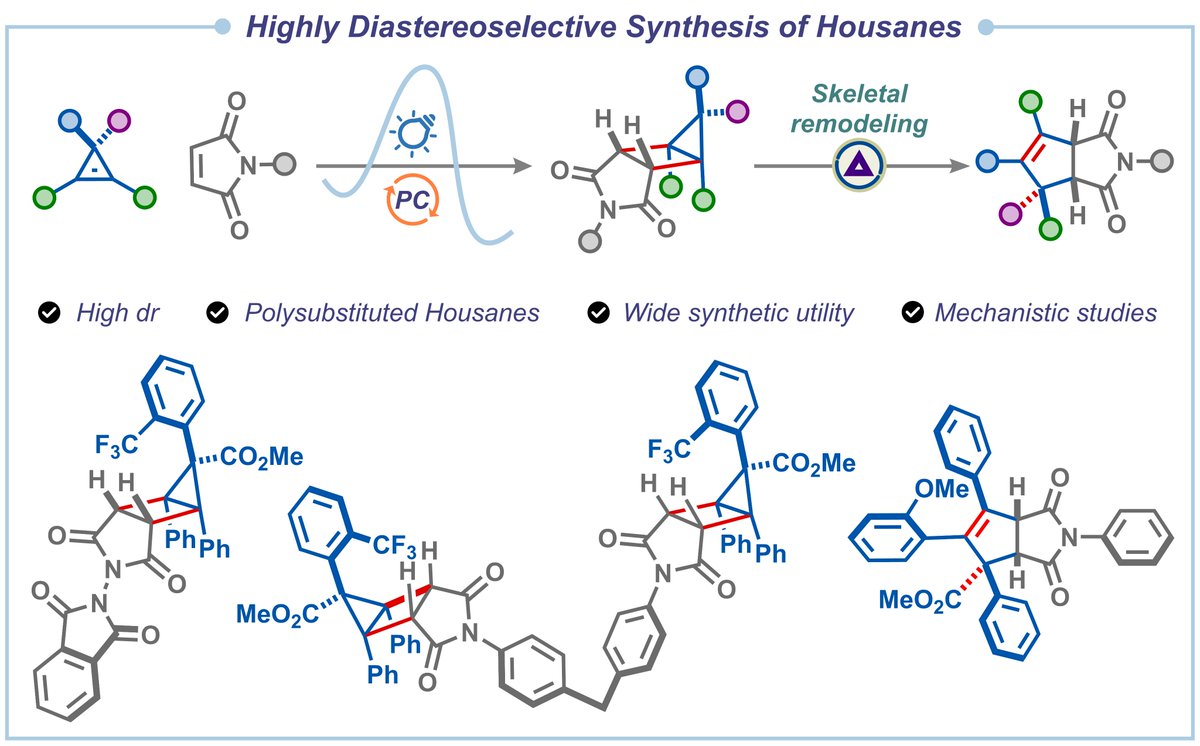
![The Hari Group (@harigroupiisc) on Twitter photo Our work on a unified strain-enabled radical-polar crossover annulation strategy to access spiro-, fused-, and enantioenriched-aza/oxa-bicyclo[3.1.1]hepatanes is out <a href="/ChemRxiv/">ChemRxiv</a>. Thank you, <a href="/iiscbangalore/">IISc Bangalore</a> <a href="/serbonline/">SERB</a>, for the financial support.
doi.org/10.26434/chemr… Our work on a unified strain-enabled radical-polar crossover annulation strategy to access spiro-, fused-, and enantioenriched-aza/oxa-bicyclo[3.1.1]hepatanes is out <a href="/ChemRxiv/">ChemRxiv</a>. Thank you, <a href="/iiscbangalore/">IISc Bangalore</a> <a href="/serbonline/">SERB</a>, for the financial support.
doi.org/10.26434/chemr…](https://pbs.twimg.com/media/Gg6nVB0awAAT64Q.jpg)