
Walter W. Chen, MD, PhD
@walterwchen1
Harvard/MIT MD-PhD + @TheBCRP grad, NICU physician @UTSWNews, postdoc @RJDLab, @statnews Wunderkind, @the_asci E-Gen awardee, studying organelles, views = mine
ID: 1044292288787214336
24-09-2018 18:27:38
4,4K Tweet
2,2K Followers
790 Following

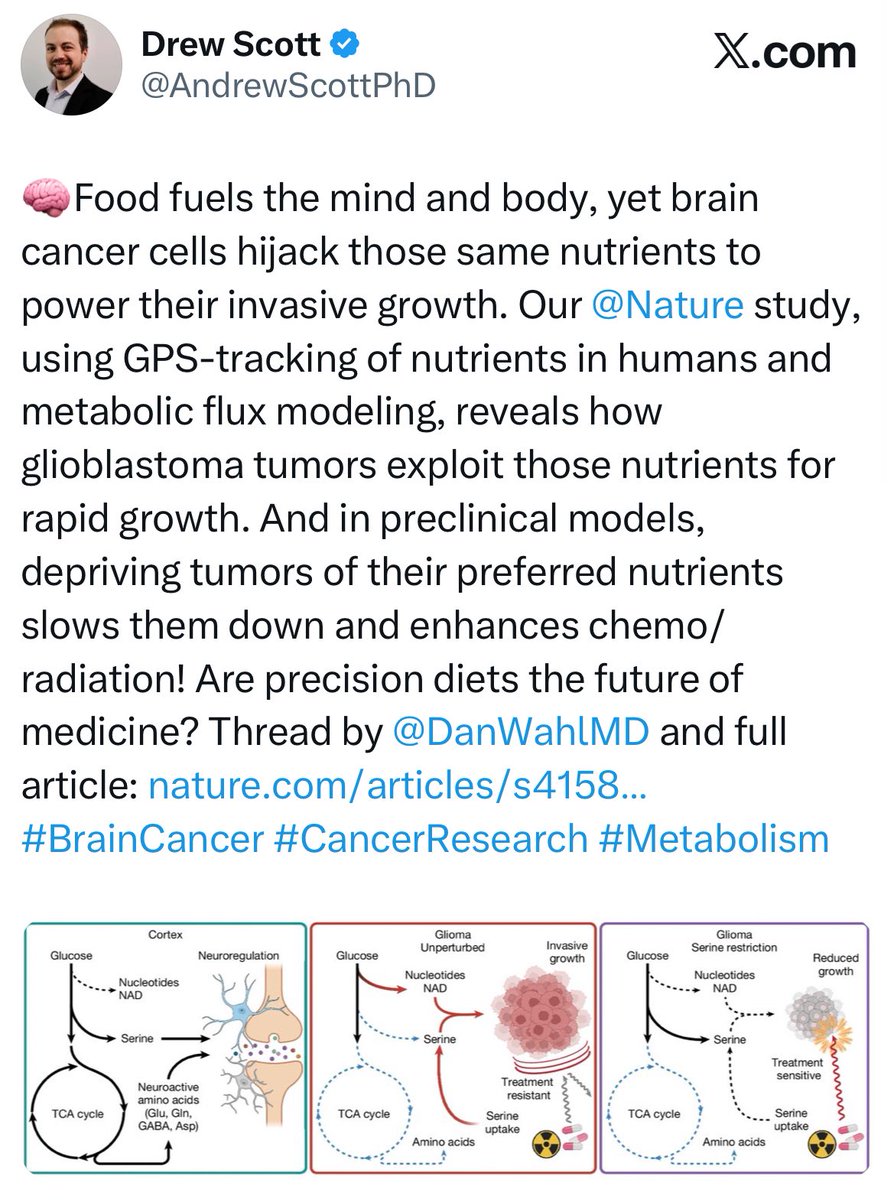
Hiring committees, please take note! Drew Scott is on the job hunt, looking for an academic home for the Scott Lab 😃 His lab will study metabolism in the brain tumor microenvironment. And if you’re into that sorta thing… he also has a K99/R00 cc: Daniel Wahl



I am excited to share my graduate work in David M. Sabatini and Dave Bartel’s labs. Since their discovery, we have known lysosomes possess RNase activity; however, their substrates were not known. Surprisingly we find specific RNAs are targeted for degradation! biorxiv.org/content/10.110…
Excited to join the Children’s Research Institute at UT Southwestern community to advance discoveries in genome integrity. We’re grateful to UTSW Department of Pathology for 6 wonderful years. 🚨 We’re recruiting motivated postdocs to study how mitotic errors and aberrant nuclear structures drive cancer genome evolution—join us!

The Baik Lab at UCSF (CVRI) is hiring a post-doc! We study hypoxia adaptation in cardiovascular disease and mechanisms of cancer therapy-related cardiotoxicities. Collaborative culture, strong mentorship, and innovative approaches! Email CV + cover letter to [email protected]

Congrats to Samir M. Parikh and team for a breakthrough paper in Science Magazine showing that somatically acquired heteroplasmic mtDNA mutations are a cause of chronic kidney disease and are functionally reversible. American Society of Nephrology ASCI 👇👇


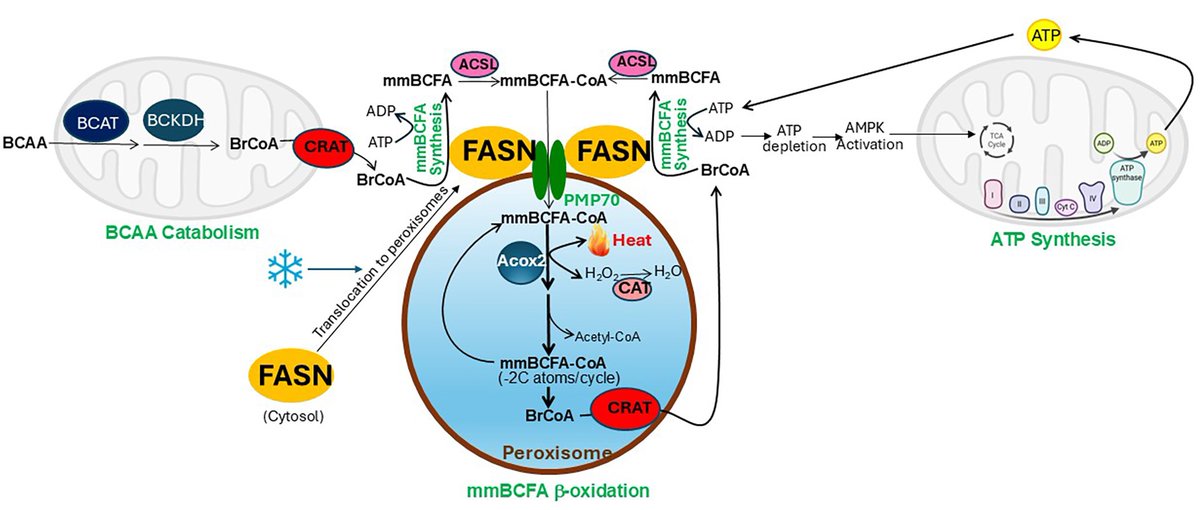
Happy to share our new paper in nature led by staff scientist Xuejing Liu Xuejing (Jessica) Liu, MD, PhD. We report identification of a UCP1-independent pathway of brown fat thermogenesis involving ATP-consuming metabolism of BCFA in peroxisomes. #Obesity #T2D nature.com/articles/s4158… WashU Medicine Department of Medicine

Wonderful to see our paper on the #organelle signatures of #neurons and #astrocytes out in final form - congratulations Shannon Rhoads and team! 🎉doi.org/10.1016/j.celr…



Grateful for the opportunity to speak at this year’s symposium on diabetes and cancer with City of Hope. Virtual meeting, registration link below #ConnectingTheDots Charles Brenner, PhD


My co-authors and I from Konnikova lab lab are very excited to present our research findings on placental immune cells! I’ll highlight some of our key findings in this thread and you can check out the full results linked below.🔬(1/n) biorxiv.org/content/10.110…


Excited to share our work with David M. Sabatini & Jonathan Weissman's Lab. How do lysosomes change with age? We present a metabolic atlas of lysosomal aging, and reveal a lysosomal “aging clock” of metabolites linked to lysosomal storage disorders. Grateful to all co-authors! biorxiv.org/cgi/content/sh…

Excited to present my research at the upcoming University of Utah’s Rising Stars Symposium and meet fellow scientists who love metabolism! Children’s Research Institute at UT Southwestern

Our new work in Cell Metabolism by Artem Khan describes three new SLC transporters, including SLC25A45, a mitochondrial transporter for methylated amino acids that is essential for carnitine synthesis and fasting adaptation. Rockefeller University cell.com/cell-metabolis…

Thank you very much, Ali! Honored and humbled to be named Thomas D. Spies Professor of Genetic Metabolism. Thanks to Shilatifard Lab Northwestern Feinberg School of Medicine Lurie Cancer Center, mentors Frédéric Bost, Brendan Manning, NoTwitterNav, my family & friends!


Thrilled to announce the launch of my lab Children’s Research Institute at UT Southwestern this January! We will explore how cells sense and respond to mechanical forces, focusing on membrane mechanics to reveal how tension and signaling work together to shape cell behavior.
