Sajan Patel
@sajancpatel
Process Chemist, PhD @StanfordUChem (Burns Group), BS @UCB_Chemistry (Tilley Group)
ID: 883907548960763904
09-07-2017 04:35:56
368 Tweet
268 Followers
399 Following

We developed a novel reaction that enables a direct synthesis of amines from olefins via C=C bond cleavage. This process which we termed Triazenolysis represents an aza-version of ozonolysis reaction. Congrats to all involved Nature Chemistry Technion Israel nature.com/articles/s4155…

'How do I thianthrenate X?' – In our new article in J. Am. Chem. Soc., Dilgam Ahmadli presents simple guidelines for selecting reaction conditions and with our collaborators from @syngenta developed a robust diversification platform for further functionalizations! pubs.acs.org/doi/10.1021/ja…


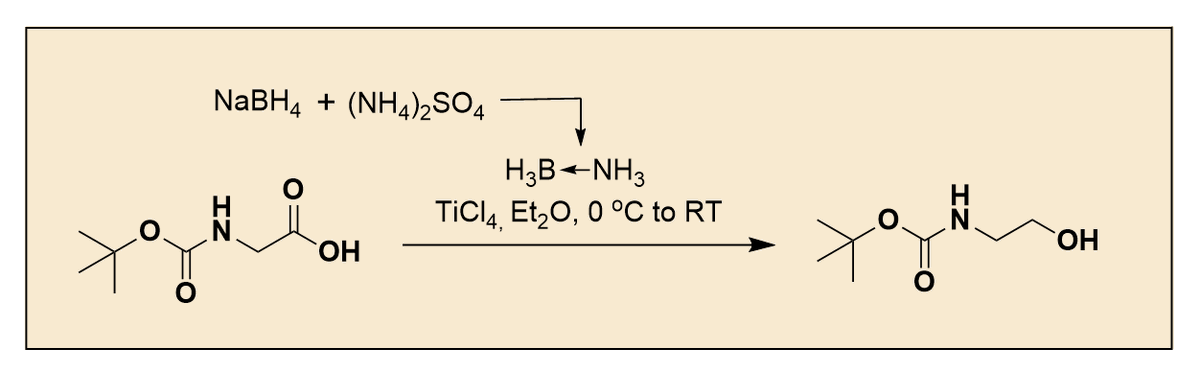
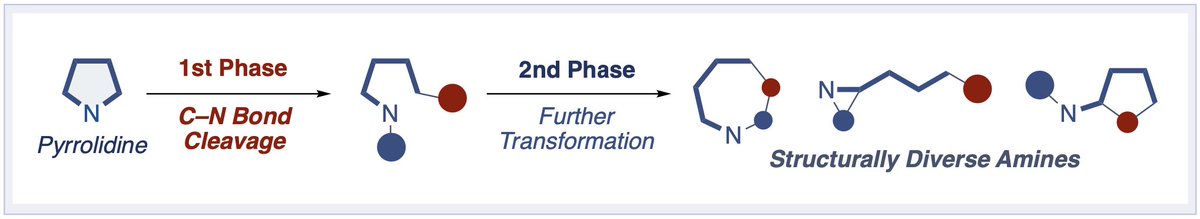
Happy to share our review on "Recent Developments for the Ring Opening of Pyrrolidines and Unstrained Cyclic Amines via C–N Bond Cleavage" in EurJOC Chemistry Europe !! Congrats to Eisuke !! doi.org/10.1002/ejoc.2…


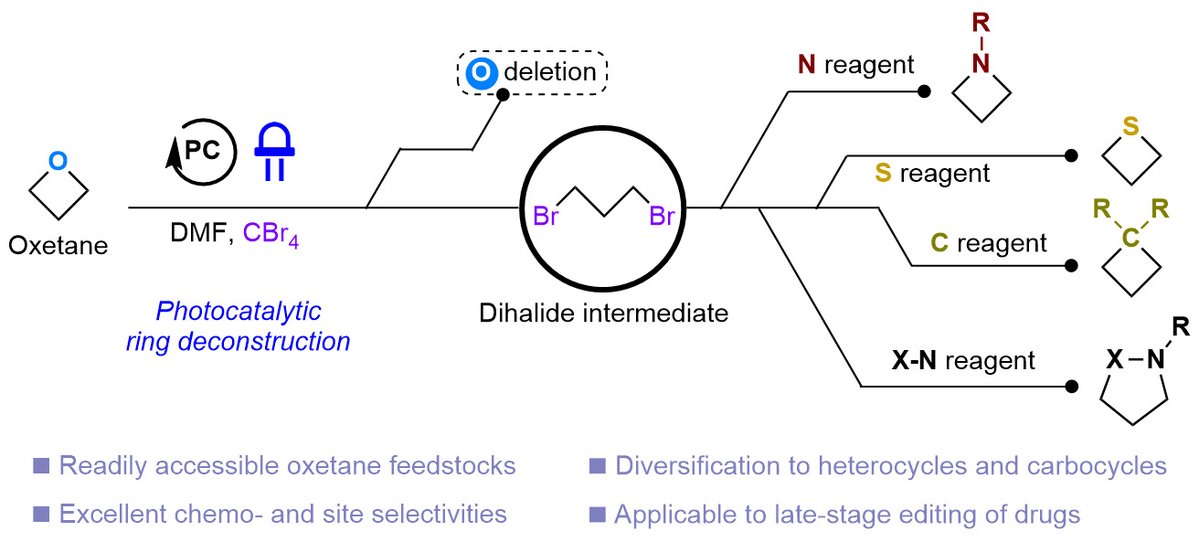
Congrats to Logan Bartholomew, Sojung Kim, Yusuke and collaborators Macroarc, Gilead Sciences, Merck on our indazole to benzimidazole conversion onlinelibrary.wiley.com/doi/10.1002/an… coordinated with Leonori Lab onlinelibrary.wiley.com/doi/10.1002/an…


Ready to take off as a synthetic chemist? Check out this tutorial review we wrote with Frank Glorius and Jasper Tyler! Glorius Group Frank Glorius

Visible Light-Promoted Deracemization of α-Amino Aldehyde by Synergistic Chiral Primary Amine and Hypervalent Iodine Catalysis | Journal of the American Chemical Society Tsinghua University Drug Discovery and Synthesis Highlights pubs.acs.org/doi/10.1021/ja…
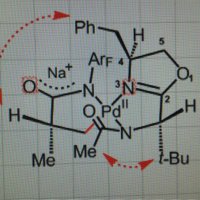

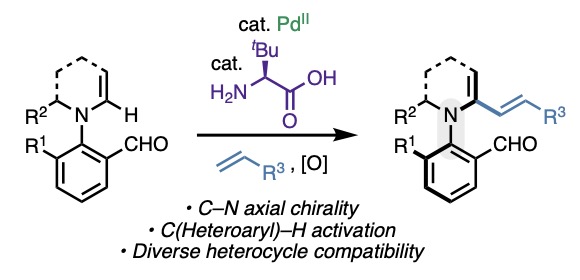
We've been drawn to selective activation of C(alkenyl)–H bonds for a long time. In a new ACS Catalysis paper, Juntao Sun & team turned attention to an interesting situation where functionalizing enamine-type azaheterocycles creates a chiral C–N axis. tinyurl.com/mpjjb99w



Check out our recent publication in J Org Chem/Org Lett on the anti-Markovnikov oxidation of alkenes enabled by photoexcited nitroarenes under iron Lewis-acid catalysis. pubs.acs.org/doi/10.1021/ac…. #photochemistry #nyuchemistry #nitroarenes

I really happy to share our lastest publication in J. Am. Chem. Soc. "Generation of Stereocenters via Single-Carbon-Atom Doping Using N-Isocyanides" Huge congrats to everyone involved! And excited to see my first paper as a corresponding author! pubs.acs.org/doi/10.1021/ja…






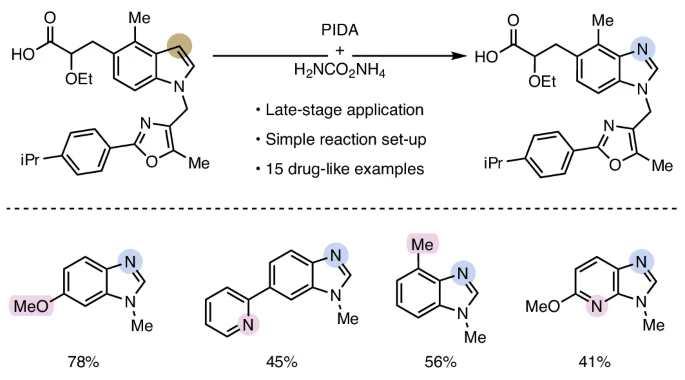
Congrats to Ann-Sophie K. Paschke, Yannick, Bence B. Botlik, Erich Staudinger, Ori Green! "Carbon-to-nitrogen atom swap enables direct access to benzimidazoles from drug-like indoles" nature.com/articles/s4155…. Nature Chemistry