Javier Pérez Ardavín
@jpardavin
Uro-oncología en H. U. i P. La Fe y andrología en Quirón Valencia, FEBU, Doctorando en último año.
ID: 546142498
05-04-2012 17:27:17
615 Tweet
516 Followers
546 Following

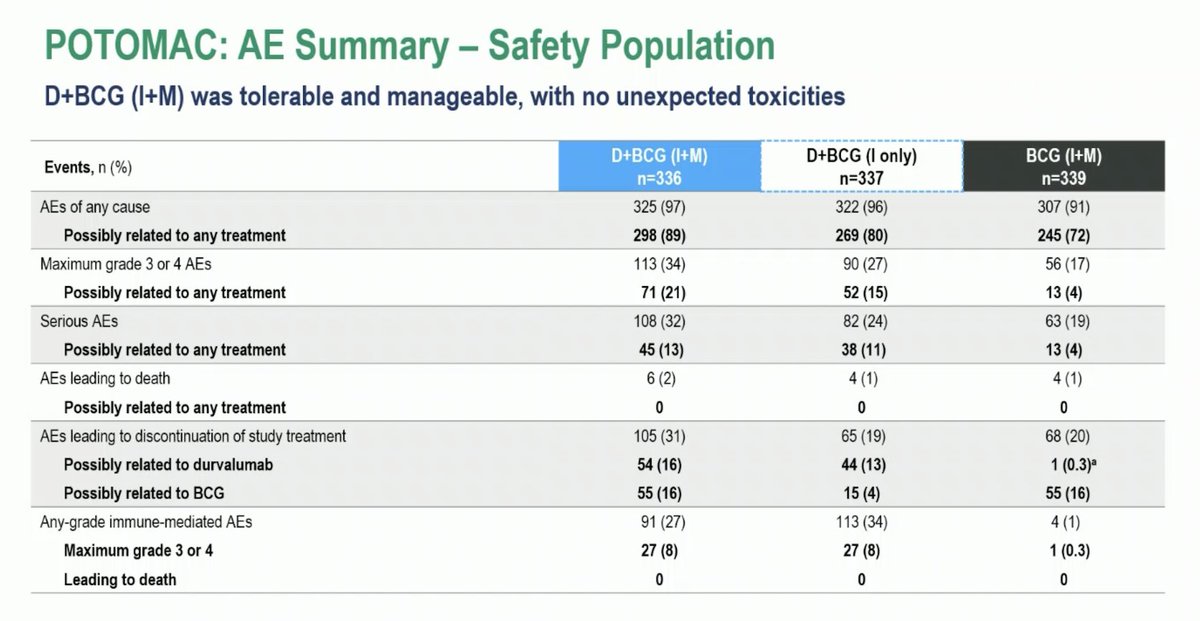
⚡️LBA⚡️POTOMAC: Ph 3 RCT of Durva + BCG for BCG-naïve HR NMIBC UroToday.com #ESMO25 Maria De Santis 🎉 Positive Trial ☑️Prim EP DFS Durva + BCG (I+M) vs BCG (I+M): HR 0.68, 95% CI 0.50–0.93 ☑️Sec EP DFS Durva + BCG (I only) versus BCG (I+M): HR 1.14, 95% CI 0.86–1.50 ☑️Gr 3/4

Another fantastic talk by Andrea Necchi #ESMO25 👉At primary analysis, intravesical TAR-200 + cetrelimab showed high pCR, pOR and 1-y RFS in MIBC #bladdercancer 👉Clearance of urinary tumor DNA portends ⬆️ rPFS👇OncoAlert Oncology Brothers Bladder Cancer Advocacy Network


Very interesting trial by Dr. Huang et al. on neoadjuvant DV + tislelizumab in UTUC as strategy for kidney sparing management! It raised more our enthusiasm for EA8192 phase 3 trial with Jean Hoffman-Censits ecog-acrin.org/clinical-trial… UTUC merits dedicated trials! OncoAlert #ESMO25 ESMO - Eur. Oncology





ARANOTE: Daro + ADT efficacy, QoL, and safety outcomes by age UroToday.com #ESMO25 🌀27% <65 yrs, 43% 65–74 yrs, 29% ≥75 yrs 🌀Daro + ADT: benefit across all age groups - rPFS - TT mCRPC - TT PSA progression - TT deterioration FACT-P 🌀TEAEs ⬆️ slightly w/ age, expected


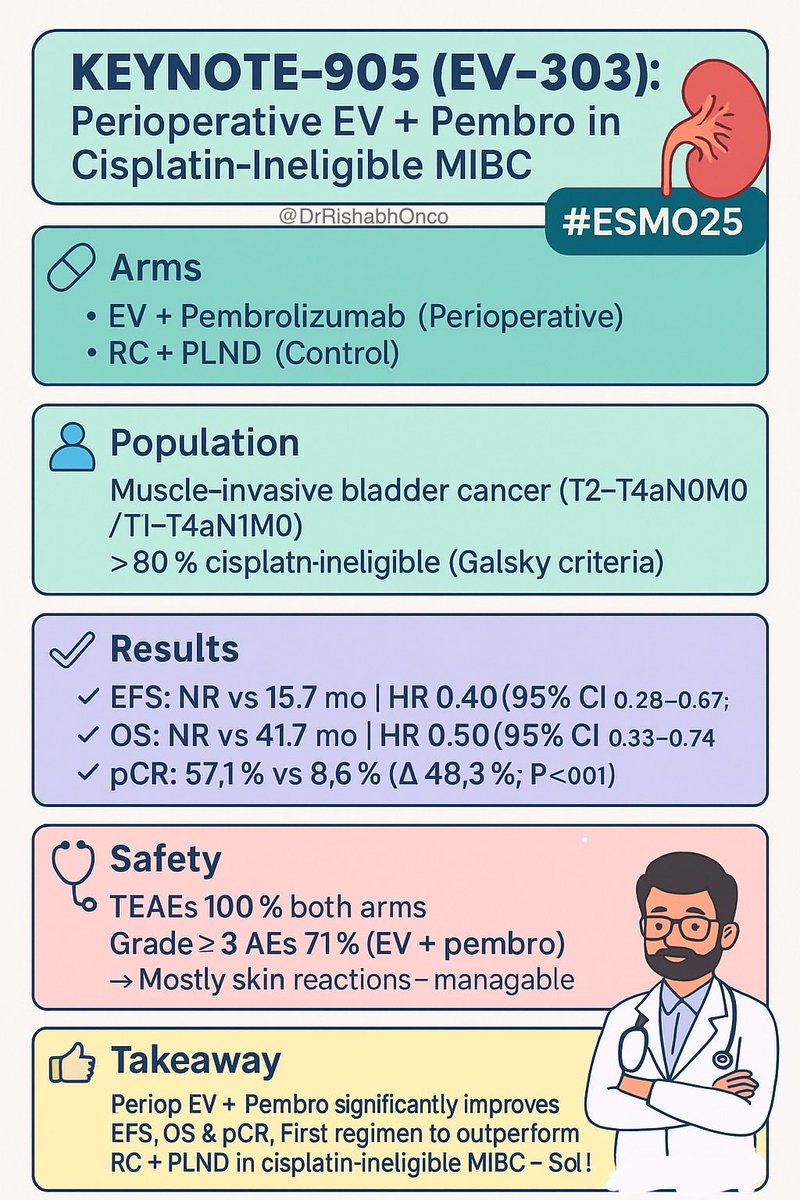
New way to treat pts with #MIBC not eligible to cisplatin presented in the plenary session at #ESMO25. EV303 showed the correct way using EV+P as perioperative strategy. The question is now...should we continue in offering surgery? Tom Powles OncoAlert GU Oncology Now Uromigos




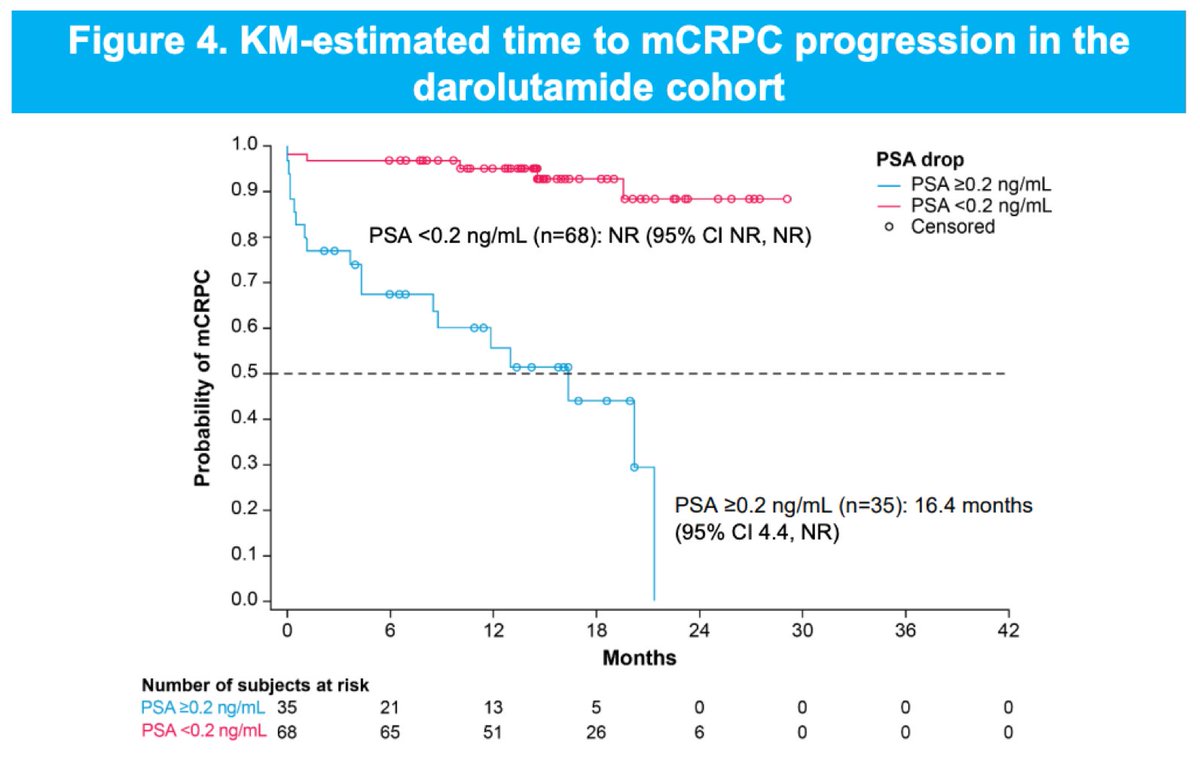
ARAAT: RW PSA resp with DARO or ABI in mHSPC triplet therapy UroToday.com #ESMO25 Alicia Morgans, MD, MPH DARO+ADT+DOCE, n=141 vs ABI+ADT+DOCE, n=101: 🌀Any time PSA <0.2: 66% vs 53% 🌀TT PSA <0.2: 6.4 vs 9.5 mos 🌀TT mCRPC (DARO): PSA <0.2 (NR) vs >0.2 (16.4 mos) 🌀Regardless of BL PSA


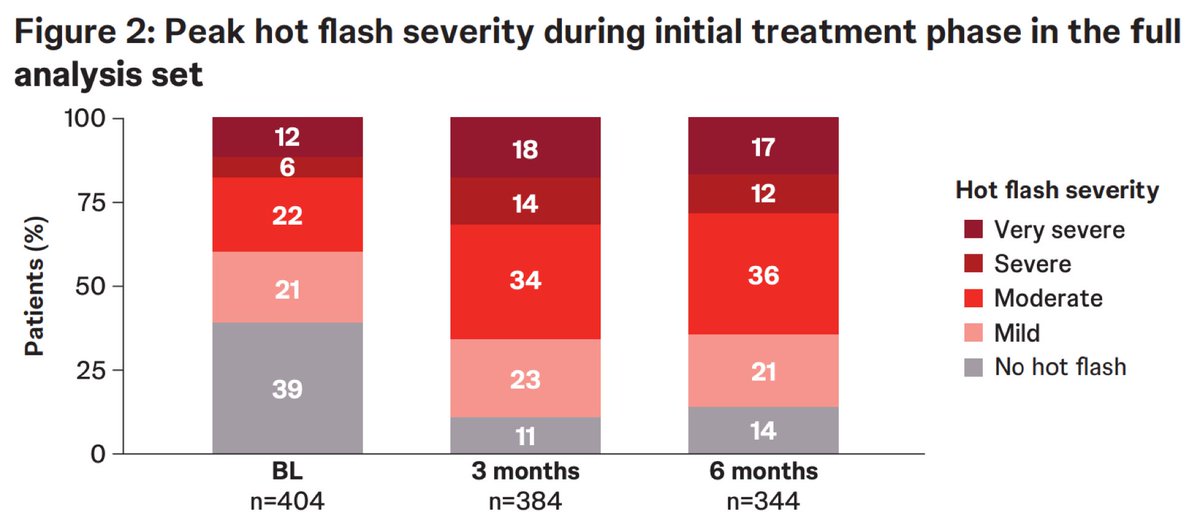
Ph 3 LIBERTAS trial in mHSPC: Initial 6 mo prevalence/severity of hot flashes (HF) UroToday.com #ESMO25 Alicia Morgans, MD, MPH 🌀BL: 59% w/ HFs 🌀HF diary compliance: 96% @ BL, 93% @ 3 mo, 97% @ 6 mo 🌀Peak HF severity: ⬆️ from BL to 6 mo 🌀HF reported as TEAE: 41% of pts; majority

Fantastic talk by Paul Nguyen #ESMO25 👉result of ph3 Enzarad trial👉Addition of ENZA to ADT + radiation did not improve MFS in all high risk localized, the primary endpoint. However, node+ on CT/MRI pts derived benefit, like Stampede trial OncoAlert UroToday.com PCF Science


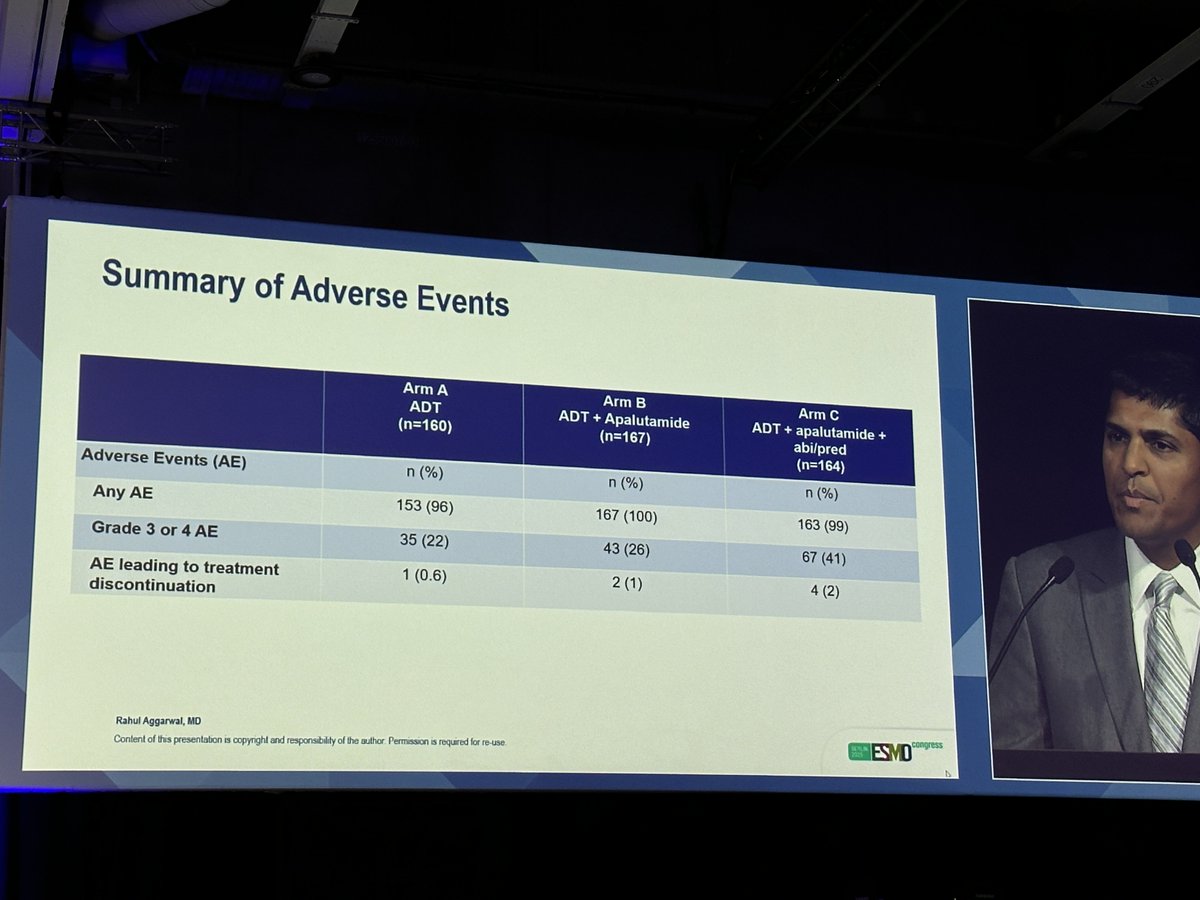
🎯 PRESTO trial: final results! (mFU 61 mo) MFS stat sign. in favor to both combo APA + ADT and APA+AA+ADT with >> g3-4 tox for triplet therapy! ESMO - Eur. Oncology OncoAlert GUARD Consortium Advanced Prostate Cancer Consensus Conference #ESMO25


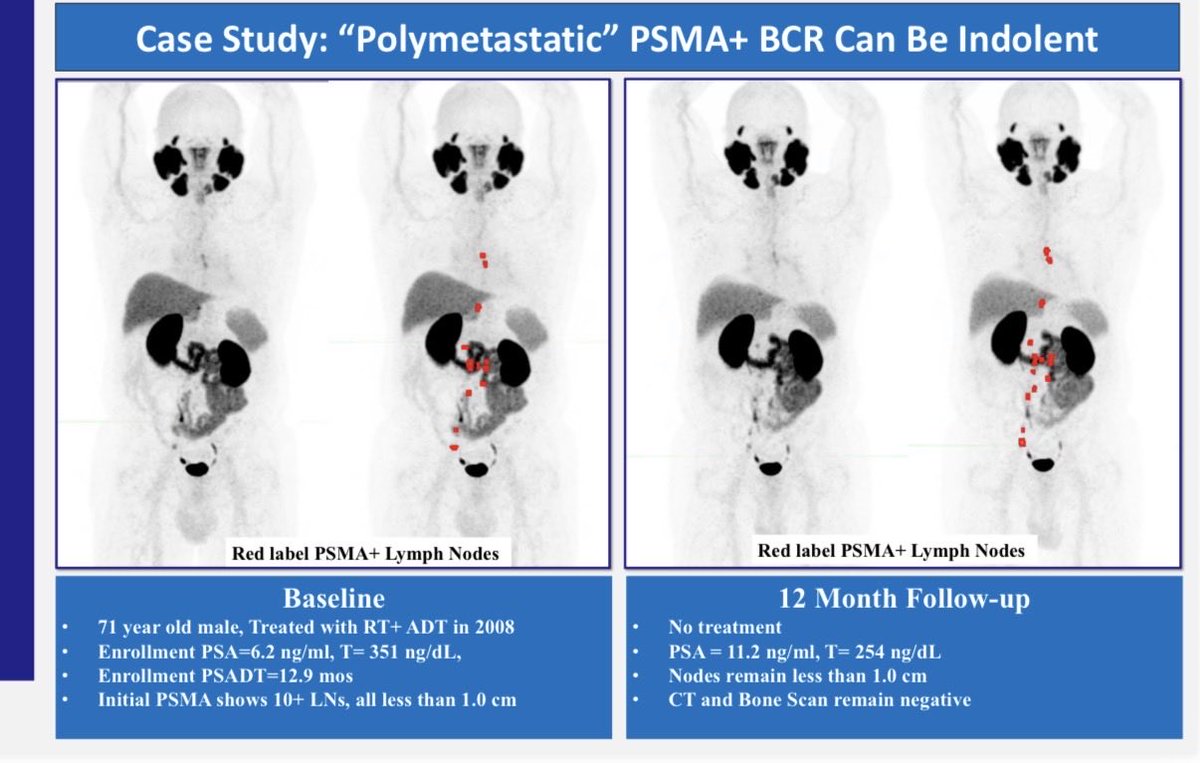
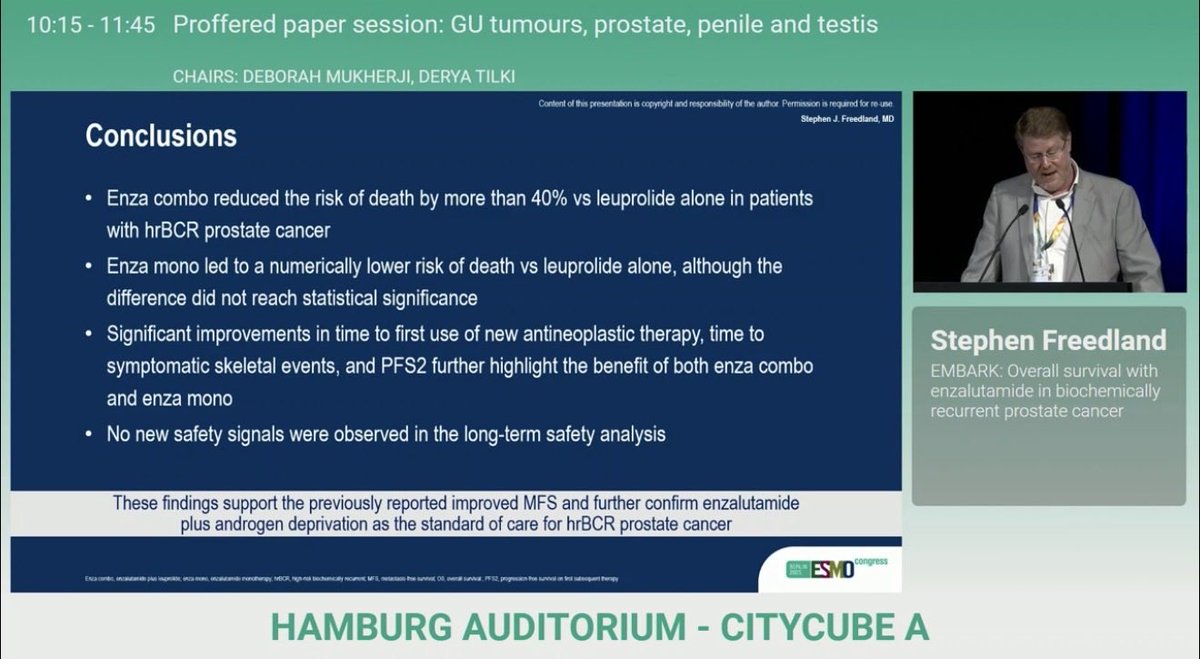
Breaking news from #ESMO25👉Improved OS in high risk biochemical recurrent #ProstateCancer on with ENZA+ADT vs ADT alone, HR: 0.59 (no OS benefit with ENZA monotherapy), 👏wonderful news for our pts👉congrats Stephen Freedland, MD neal shore & the team. OncoAlert UroToday.com PCF Science

Drug Approvals in 🇺🇸 for Prostate Cancer (2010-2025) Go ahead and bookmark this beauty from Pedro C Barata, MD MSc FACP UroToday.com #ESMO25


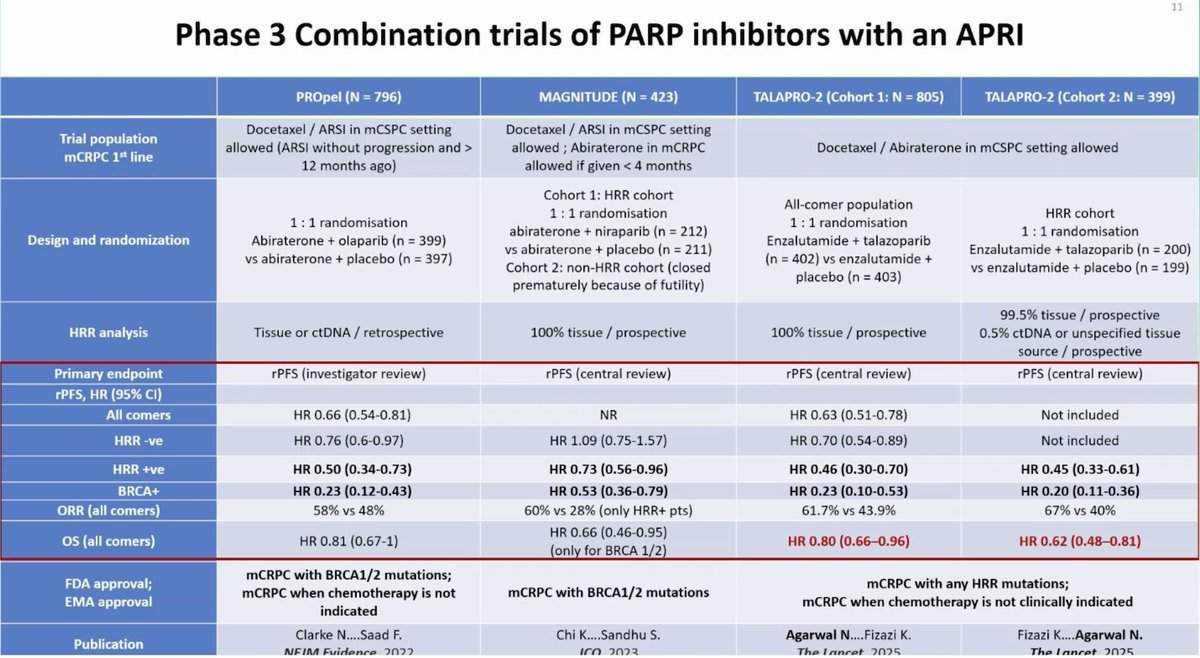
🚨Comprehensive summary of Phase 3 Combination Trials of PARP inhibitors with an ARPI from Neeraj Agarwal, MD, FASCO UroToday.com #ESMO25

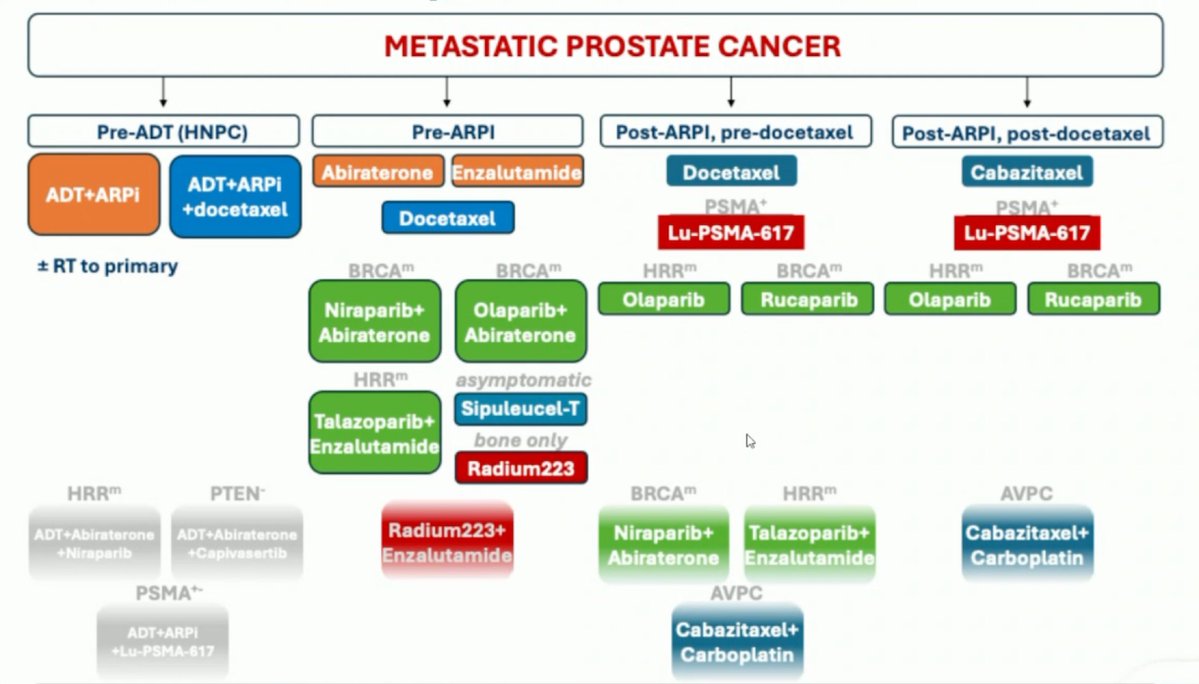
Another great metastatic prostate cancer "landscape" figure from Ana Aparicio during her aggressive variant prostate cancer talk at #ESMO25 Approved, and "likely to be approved"/new data (bottom), treatment options UroToday.com