
Simon Stern
@drsstern
Consultant Haematologist. Interested in haemato-oncology in general and multiple myeloma in particular.
ID: 3082842788
15-03-2015 00:05:40
1,1K Tweet
331 Followers
292 Following


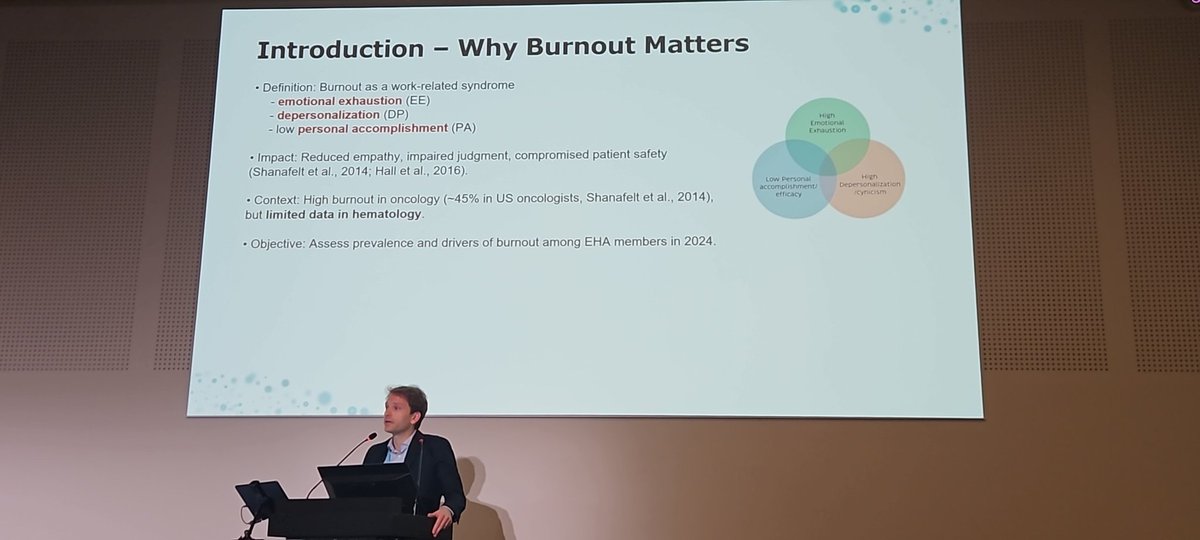
Really important session by Come Bommier discussing burnout amongst haematologists #EHA2025 European Hematology Association

CONGRESS | #EHA2025 | PRESENTATION Shaji Kumar Shaji Kumar Mayo Clinic presents results from the phase II RedirecTT-1 trial of talquetamab + teclistamab in patients with RRMM with EMD. Tal + Tec led to an ORR of 78.9% and a ≥CR rate of 54.4%; efficacy exceeded standard therapies,



Somewhat strange to hear Nick Robinson haranguing an NHS Consultant for being on “over £100,000” and claiming to channel the feelings of ordinary people. His salary in 2024 was between £345,000 - £349,000. For presenting radio programs. Which never saved anyone’s life?


Nelson Mandela's World Cup moment was 30 years ago on Tuesday. Inequality hasn't shifted in South Africa but the magic of that day endures. My column for The Observer observer.co.uk/news/sport/art…



Do we need maintenance therapy for everyone in quadruplet era? particularly relevant for older adults with CEPHEUS and IMROZ results. lucky me that I got to write this with Luciano J Costa 😃 onlinelibrary.wiley.com/doi/full/10.10… McMaster University





Thanks to Robert Z. Orlowski for highlighting a further publication from the iTIMM study, led by Christina Messiou The Royal Marsden NHS Foundation Trust. MRD detected by whole body MRI clearly predictive of PFS post ASCT. Martin Kaiser

Just out! Current Risk stratification of all Plasma Cell Disorders. MGUS, SMM, Myeloma, Amyloidosis. We paid Open Access fees so you can download and read freely! Lots of Tables. Bookmark. Saurabh Zanwar Shaji Kumar Leukemia Journal Link: nature.com/articles/s4137…


🚨 NEWS 🚨 European Commission grants approval to subcutaneous daratumumab monotherapy for the treatment of high-risk smoldering #MultipleMyeloma; based on data from the phase III AQUILA study. This approval represents the first authorized treatment in this indication. Read more:






![Robert Z. Orlowski (@myeloma_doc) on Twitter photo #Myeloma Paper of the Day: Simultaneous whole-body multi-parametric 2-[18F]FDG-PET/MRI in smoldering myeloma assessment showing presence of diffuse BM involvement on MRI was the strongest adverse prognostic parameter for PFS to symptomatic myeloma: pubmed.ncbi.nlm.nih.gov/40775455/. #mmsm #Myeloma Paper of the Day: Simultaneous whole-body multi-parametric 2-[18F]FDG-PET/MRI in smoldering myeloma assessment showing presence of diffuse BM involvement on MRI was the strongest adverse prognostic parameter for PFS to symptomatic myeloma: pubmed.ncbi.nlm.nih.gov/40775455/. #mmsm](https://pbs.twimg.com/media/Gx0ki3DXYAAO-pB.png)