Elena K. Berg
@elena_k_berg
MD | Urology resident | @LMU_Muenchen | LMU Prostate Cancer Center
ID: 1482290636913053697
15-01-2022 09:56:49
6 Tweet
45 Followers
124 Following




ENZA-P in The Lancet Oncology Lu-177 PSMA-617 + enzalutamide vs. enza alone: ● ↑PFS: 13.0 vs 7.8, hazard radio 0.43 ● PSA90 response 78% vs 37% ● PSA50 response 93% vs. 68% ● no additional side effects Incredible work from Louise Emmett Ian Davis & ANZUP team
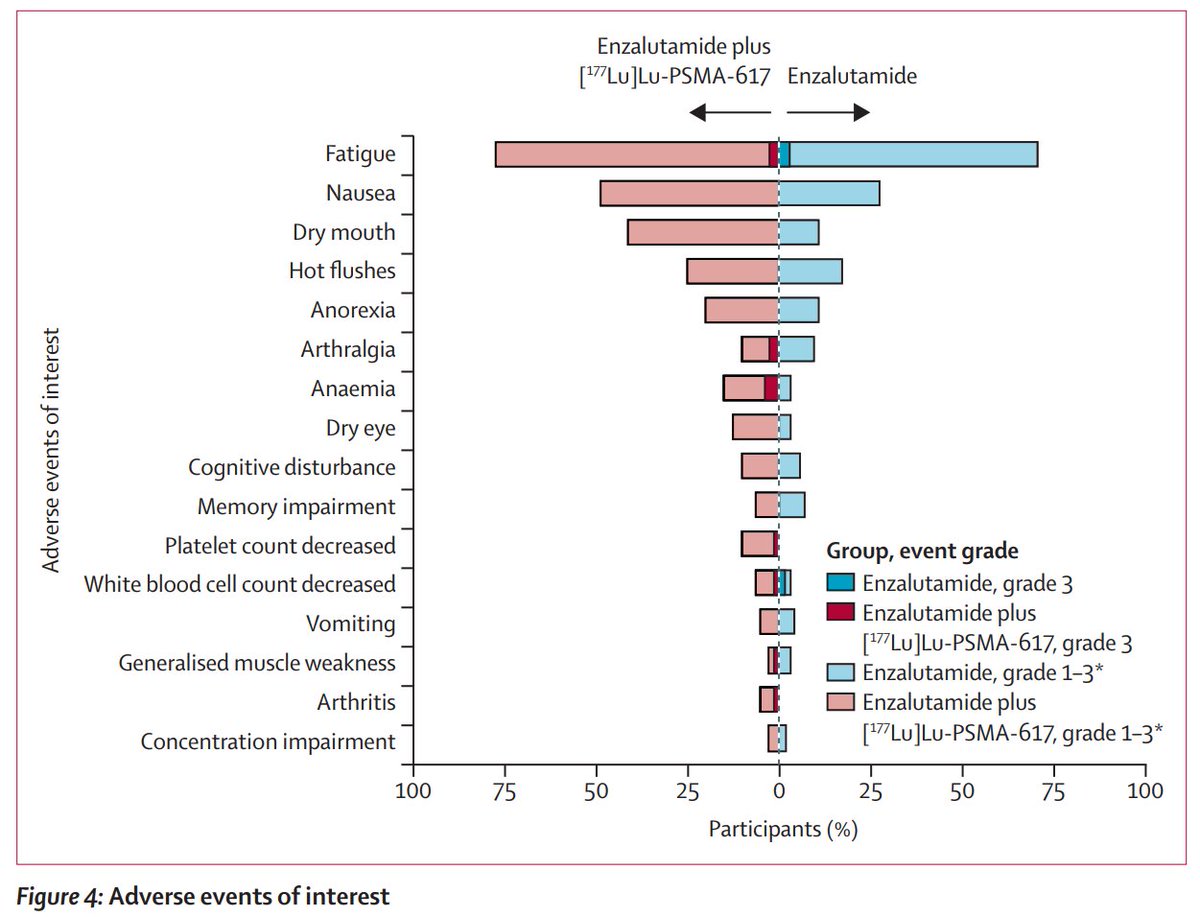


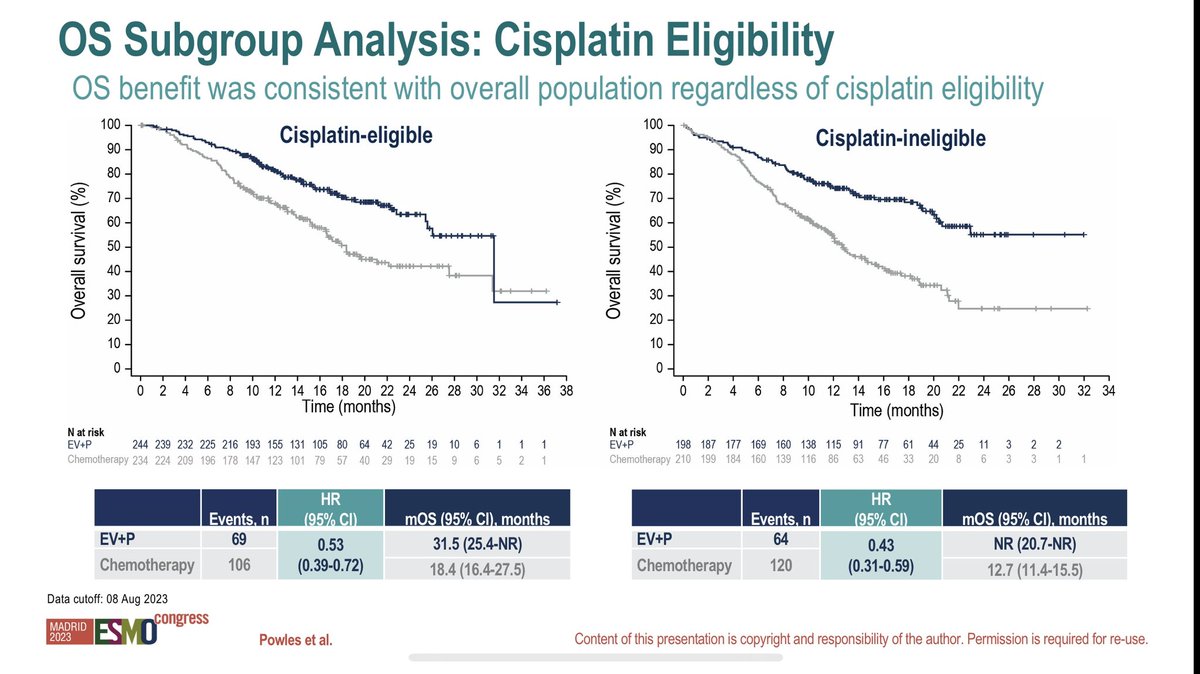
Outstanding talk from silke gillessen elegantly presenting PEACE-3 phase 3 trials first results. Adding 6 cycles of Ra223 to enzalutamide as first-line therapy for mCRPC showed significant rPFS improvement + significant OS benefit #ESMO24 ESMO - Eur. Oncology OncoAlert UroToday.com