John D. Helmann
@johnhelmann
Professor. Bacillus subtilis physiology and stress responses (metal homeostasis, redox stress, antibiotic resistance & cell envelope). EIC of Mol Microbiol.
ID: 2475315025
03-05-2014 10:50:27
1,1K Tweet
2,2K Followers
803 Following

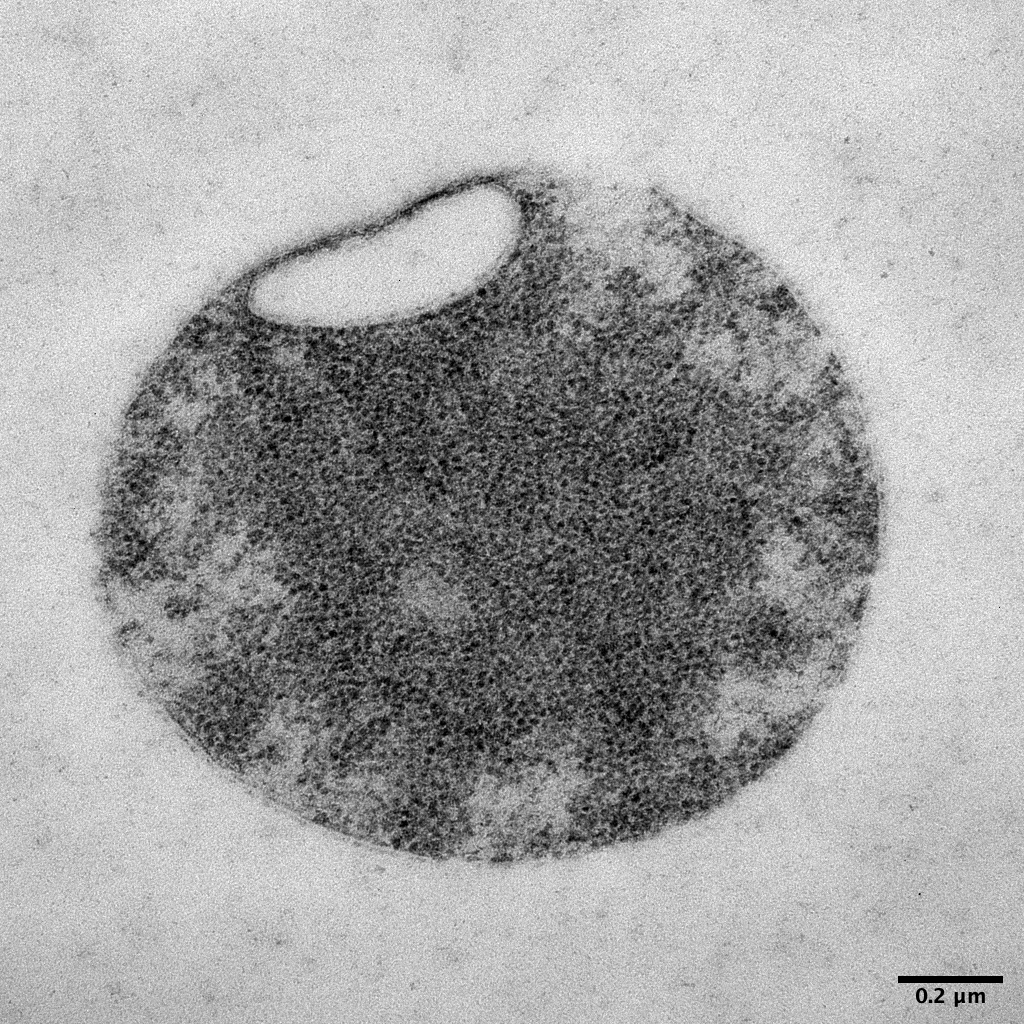
A family of DNA glycosylases in bacteria that repairs interstrand DNA crosslinks, protects against acid, and is involved in infection. New work from Dillon Kunkle and YUJUAN CAI in collaboration with Brandt Eichman PNASNews pnas.org/doi/10.1073/pn…



A huge step in understanding the biological role of flotillin and membrane microdomains. Led by our very own Marta Ukleja 🇵🇱🙌🙌🙌 with the help of CavaLab, Mateus lab and (twitterless) Ana Eulalio :-). CaixaResearch funding. rdcu.be/dMWvC



I’ve felt more than lucky to be able to tour around the US! Thanks to John D. Helmann and his whole lab for being so welcoming and talking some great science (and not science!) with me this week 🧬👩🔬


onlinelibrary.wiley.com/doi/10.1111/mm… BLAST Meetings Mol Micro Editors

Membrane depolarization kills dormant Bacillus subtilis cells by generating a lethal dose of ROS Nature Communications from Leendert Hamoen@HamoenLab with Henrik Strahl @KursadTurgay nature.com/articles/s4146…

Our new paper out in PLOS Pathogens today: dx.plos.org/10.1371/journa… We find that resistance/tolerance to antimicrobial peptides is slightly more complex and dynamic than your "average" antibiotic resistance. Nice work by Andrew Murtha et al.!

Read the new story of Robert Warneke (inactive) on coenzyme A biosynthesis in Bacillus subtilis and the identification of a novel metabolite, cysteinopantetheine! Excellent collaboration with Christoph Elfmann @IFeussner and teams! mBio Uni Göttingen #subtiwiki journals.asm.org/doi/10.1128/mb…

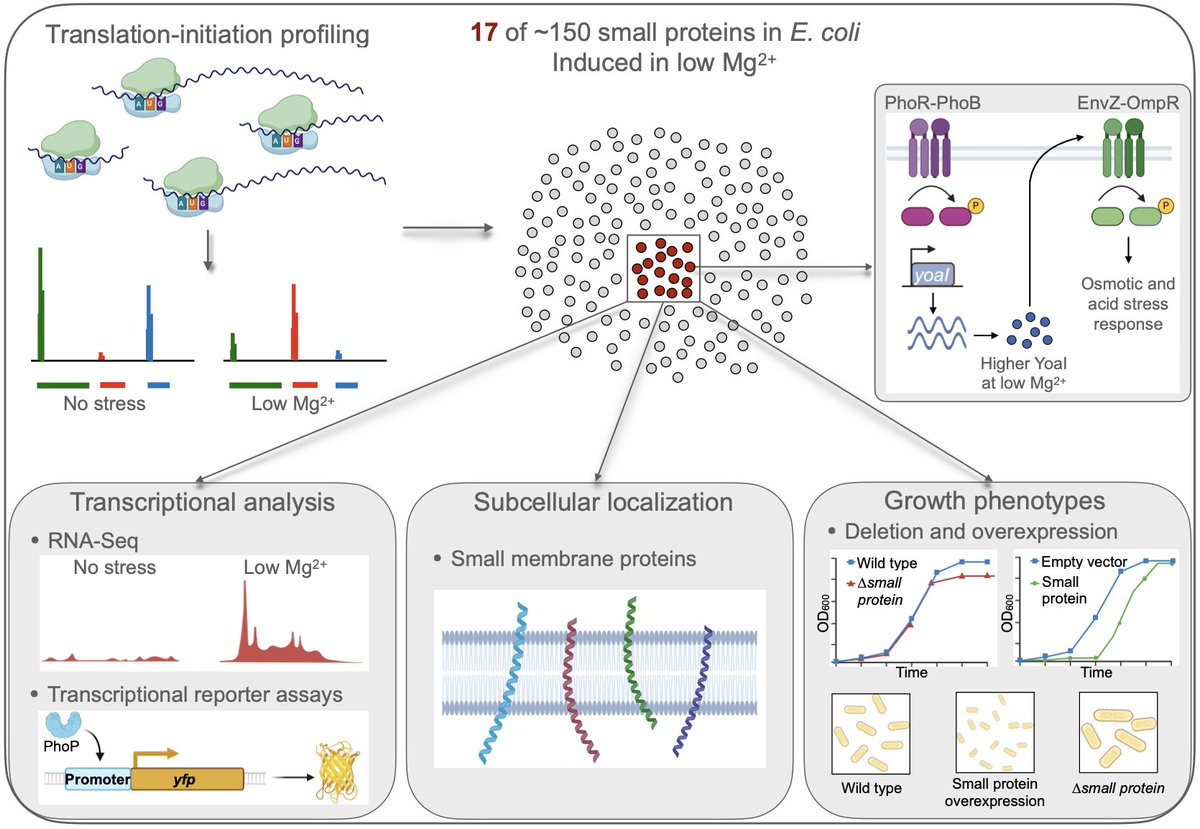
🧵 Exciting new findings on bacterial stress response regulation, driven by small proteins (≤50 amino acids). Big shoutout to the amazing team behind this work led by Sangeevan Vellappan (Sangeev). Read the full study here: biorxiv.org/content/10.110…


A new study in PNAS explores the connection between methionines and copper, with a focus on CueO. A collaboration between @lcb_officiel and @BIP8_Marseille 🍾🍾 Methionine-rich domains emerge as facilitators of copper recruitment in detoxification systems pubmed.ncbi.nlm.nih.gov/39378088/


Curious about organelle biogenesis in bacteria 🦠🔬Check out our review on bacterial organelles and their role in iron physiology Mol Micro Editors doi.org/10.1111/mmi.15…