Cisema
@cisemahk
Regulatory Affairs & CRO in China for Life Sciences, Cosmetics, Health Foods, Industrial & Consumer Goods
ID: 1262937137130795013
http://cisema.com 20-05-2020 02:44:23
307 Tweet
235 Takipçi
955 Takip Edilen













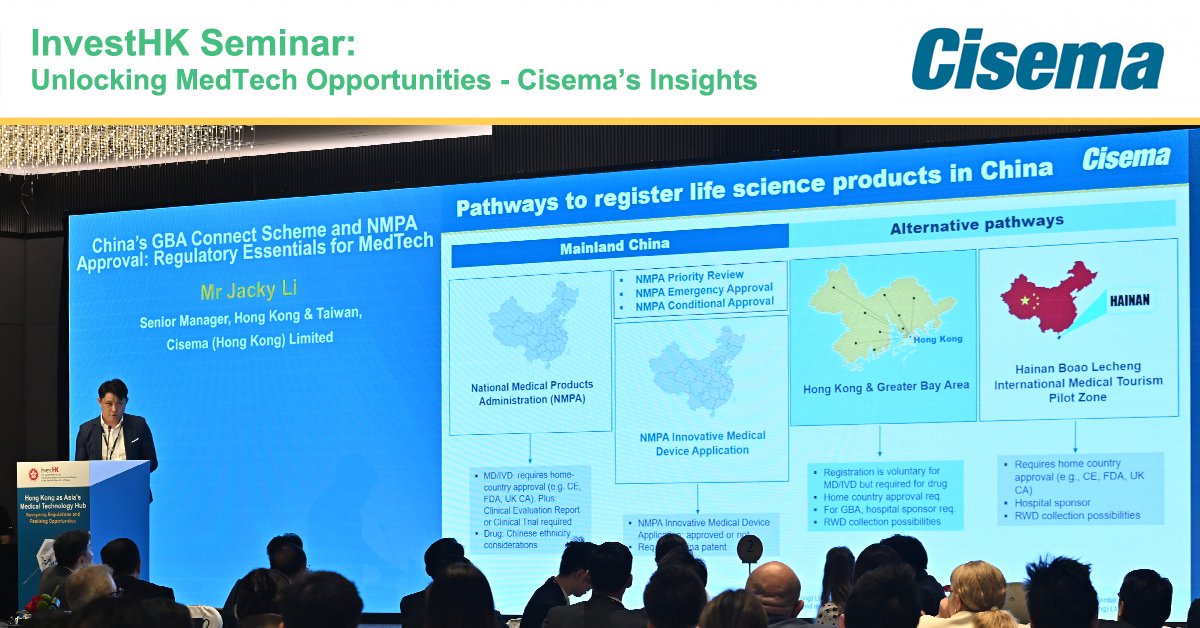
Earlier this month, Jacky Li joined the InvestHK HKSAR seminar on Hong Kong's role as a medtech hub and gateway to China via the GBA. Key takeaways and insights here: lnkd.in/dBpBuiGc #MEDTECH #HongKong #Regulatory