Vision-DMD Project
@vision_dmd
Project aiming to advance clinical development of orphan drug Vamorolone for treatment of DMD.
ID: 784352898230718464
http://www.vision-dmd.info 07-10-2016 11:21:18
217 Tweet
487 Followers
144 Following





Thanks #EUfunded @EJPRareDiseases research co-fund for advertising this White Paper of #EUfunded research Vision-DMD Project project: returning individual clinical trial results back to patients - important not only for rare disease patients ! Horizon 2020 #ResearchImpactEU




The power of collaboration: Positive results in VISION DMD study funded by #Horizon2020, #DMD #PatientGroups and US Gov – we are now entering the final phase towards Market Authorisation @santhera ReveraGen BioPharma EU Research Results EU Science & Innovation 🇪🇺 #WDAD2021



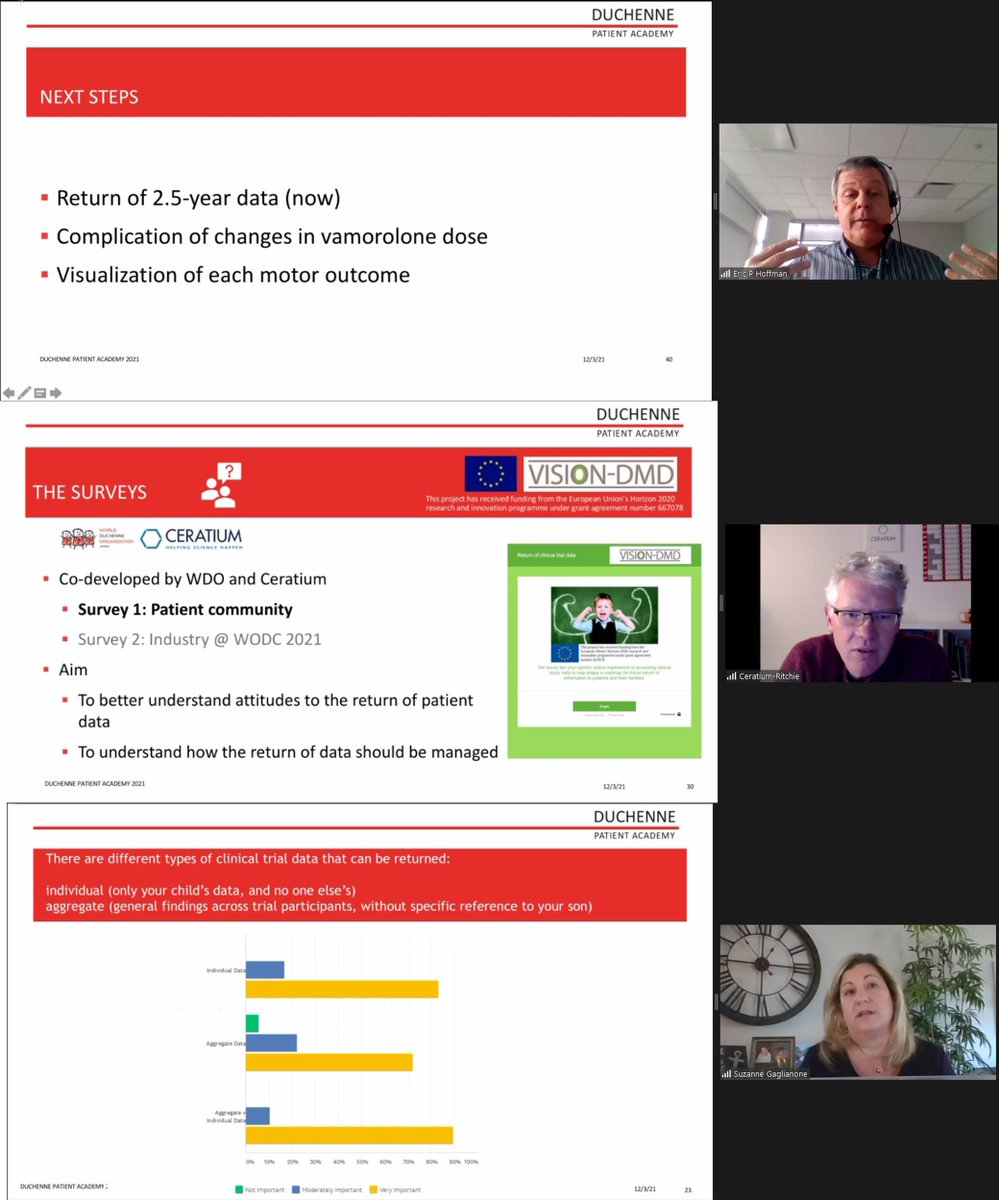
💬 What is the patient perspective on returning individual clinical trial data back to participants after a trial has ended? Help Vision-DMD Project answer this question. Please complete this short 4-minute survey 👇 worldduchenne.org/news/return-of… #Duchenne #ClinicalTrial #HealthData




Throughout the study, Vamorolone: → Showed sustained efficacy across multiple endpoints over 48 weeks → Good safety and tolerability profile was confirmed More about the topline results from the Vision-DMD Project study 👇 worldduchenne.org/news/topline-r…

Eric Hoffman, Suzanne Gaglianone Suzanne Gaglianone from ReveraGen BioPharma and Ritchie Head from Ceratium share their experience on what Patient Organizations can do to have data return from clinical trials #DPA2021 #duchenne