TiefenbacherLab
@tiefenbacherlab
Student-run account for the Tiefenbacher group
ID: 786902257216450560
https://nanocat.chemie.unibas.ch/en/ 14-10-2016 12:11:33
35 Tweet
668 Followers
168 Following

Supramolecular catalysts (capsules, anion-π, …) compared for asymmetric autocatalysis, self-replication, and breaking of the Baldwin rules – @NCCR_MSE teamwork makes it possible TiefenbacherLab Université de Genève Faculty of Science | UNIGE @NCCR_ChemBio
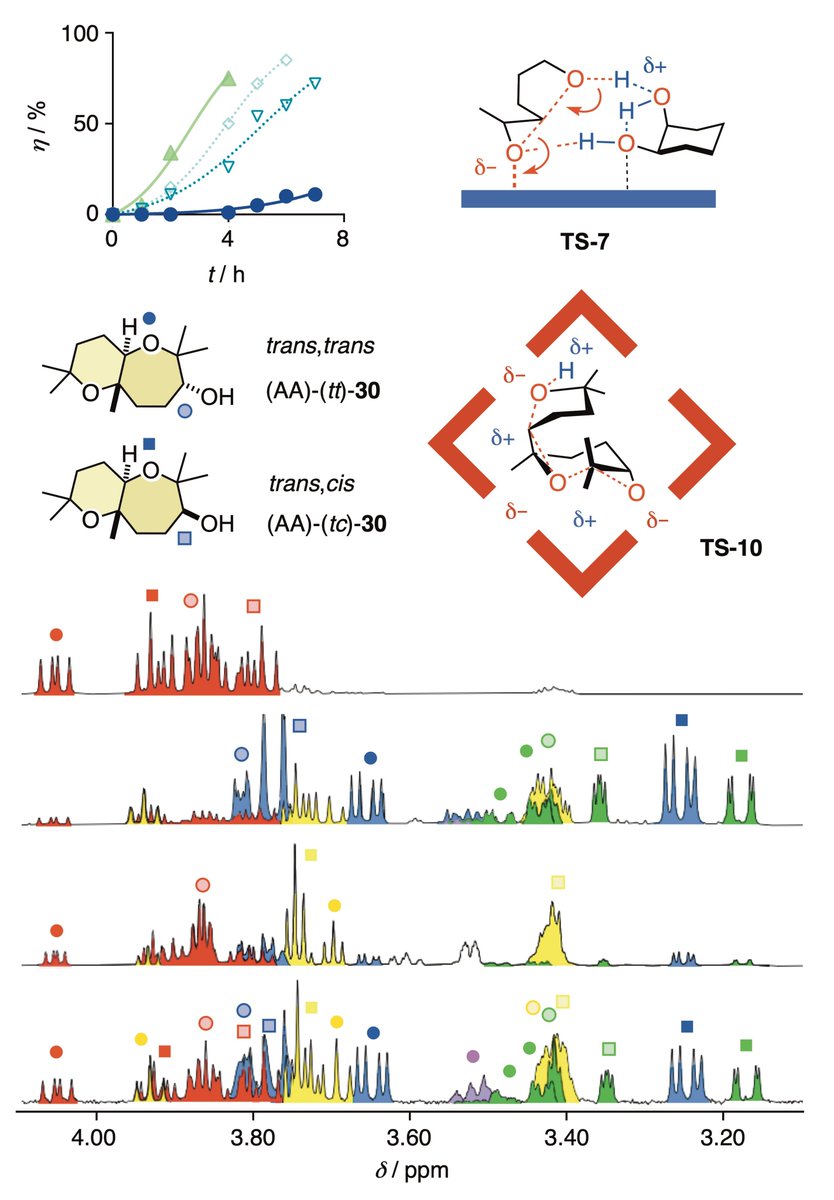

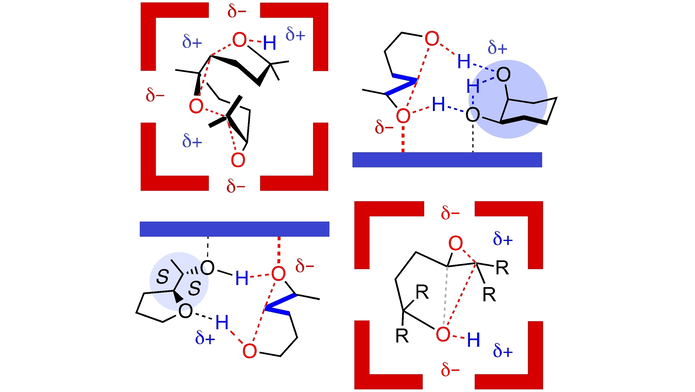
We report the resorcin[4]arene capsule as an efficient catalyst for epoxide-opening ether cyclizations. A comparison with alternative catalysts revealed a unique, product selectivity. A great @NCCR_MSE collaboration with the group of Prof. Pierre Matile. …mistry-europe.onlinelibrary.wiley.com/doi/abs/10.100…

Bioinspired ether cyclizations within a (pi)-basic capsule compared to autocatalysis on (pi)-acidic surfaces and pnictogen-bonding catalysts (TiefenbacherLab The Matile Group UNIGE_en) #OpenAccess onlinelibrary.wiley.com/doi/10.1002/ch…

We report an optimized version of our previously published iminium-catalysed 1,4-reductions inside the resorcinarene capsule. The enantioselectivity was increased to >90% ee through the addition of alcohol additives. RSC Advances Royal Society of Chemistry pubs.rsc.org/en/content/art…

Thioderivatives of Resorcin[4]arene and Pyrogallol[4]arene: Are Thiols Tolerated in the Self-Assembly Process? A study in #OL on the influence of free thiols on self-assembly, disulfide chemistry, and catalysis. From TiefenbacherLab. Check it out: fal.cn/3hIQd
![J Org Chem/Org Lett (@joc_ol) on Twitter photo Thioderivatives of Resorcin[4]arene and Pyrogallol[4]arene: Are Thiols Tolerated in the Self-Assembly Process? A study in #OL on the influence of free thiols on self-assembly, disulfide chemistry, and catalysis. From <a href="/TiefenbacherLab/">TiefenbacherLab</a>. Check it out: fal.cn/3hIQd Thioderivatives of Resorcin[4]arene and Pyrogallol[4]arene: Are Thiols Tolerated in the Self-Assembly Process? A study in #OL on the influence of free thiols on self-assembly, disulfide chemistry, and catalysis. From <a href="/TiefenbacherLab/">TiefenbacherLab</a>. Check it out: fal.cn/3hIQd](https://pbs.twimg.com/media/E9stYP4WYAQO5pf.jpg)

We Report the synthesis of new thioderivatives of Resorcin[4]arene and Pyrogallol[4]arene and a study of their properties with regard to self-assembly, disulfide chemistry, and Brønsted acid catalysis. J Org Chem/Org Lett ACS Publications pubs.acs.org/doi/10.1021/ac…

Congratulations Dr. Pham – outstanding discussion with TiefenbacherLab, wonderful to be back live, with hybrid participation from all over the world UniGE - Organic Chemistry Department Faculty of Science | UNIGE Université de Genève @NCCR_ChemBio @NCCR_MSE



We report that the resorcinarene capsule/HCl-catalyzed carbonyl olefin metathesis tolerates Lewis-basic protecting groups, in contrast to most alternative catalysts. pubs.acs.org/doi/10.1021/ac… J Org Chem/Org Lett ACS Publications

We report the first examples of optically active hexameric resorcin[4]arene capsules, which catalyze the enantioselective tail-to-head terpene cyclization of nerol with e.e. of up to 70%. Angewandte Chemie onlinelibrary.wiley.com/doi/10.1002/an…


Megalo-Cavitands: Synthesis of Acridane[4]arenes and Formation of Large, Deep Cavitands for Selective C70 Uptake by TiefenbacherLab #openaccess onlinelibrary.wiley.com/doi/10.1002/an…
![Angewandte Chemie (@angew_chem) on Twitter photo Megalo-Cavitands: Synthesis of Acridane[4]arenes and Formation of Large, Deep Cavitands for Selective C70 Uptake by <a href="/TiefenbacherLab/">TiefenbacherLab</a> #openaccess onlinelibrary.wiley.com/doi/10.1002/an… Megalo-Cavitands: Synthesis of Acridane[4]arenes and Formation of Large, Deep Cavitands for Selective C70 Uptake by <a href="/TiefenbacherLab/">TiefenbacherLab</a> #openaccess onlinelibrary.wiley.com/doi/10.1002/an…](https://pbs.twimg.com/media/FcNzVLrWQAM50s7.png)

Hyperresponsive expanding XL product space? Well then, never mind, @NCCR_MSE spirit at its best, amazing how these magic TiefenbacherLab capsules make new molecules, how pnictogen-bonding catalysis breaks the Baldwin rules. Thank you Chemical Science Université de Genève Faculty of Science | UNIGE






