Matthew Murray, Ph.D.
@mattamurrayy
Biotech Equity Research Associate at Jones Research | Yale and FSU Alum | he/him | views are my own
ID: 1123950933027823616
https://www.linkedin.com/mwlite/in/matthew-a-murray 02-05-2019 14:02:39
608 Tweet
210 Followers
613 Following

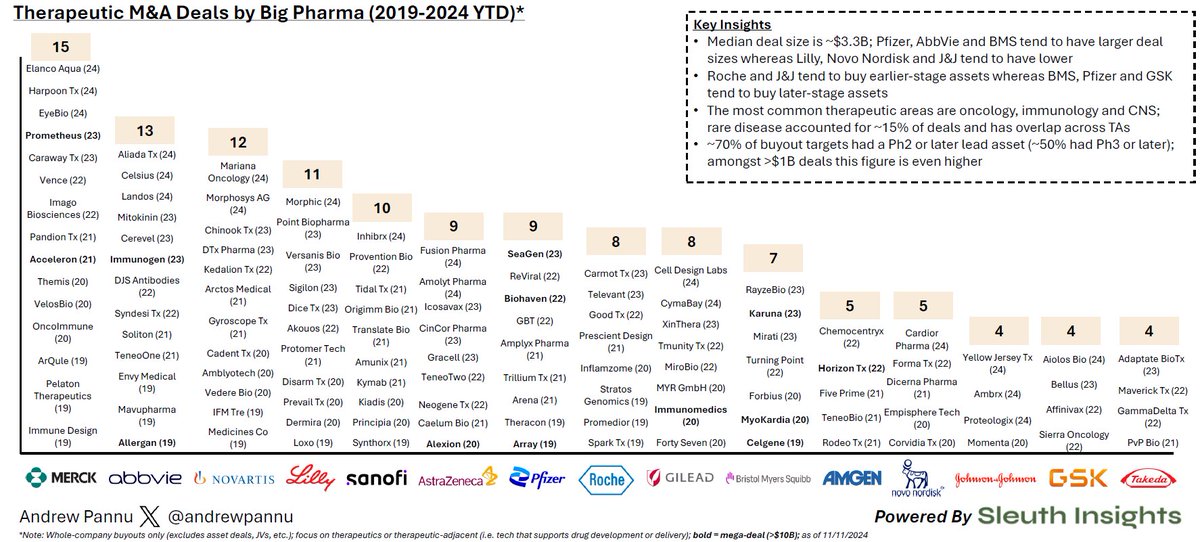
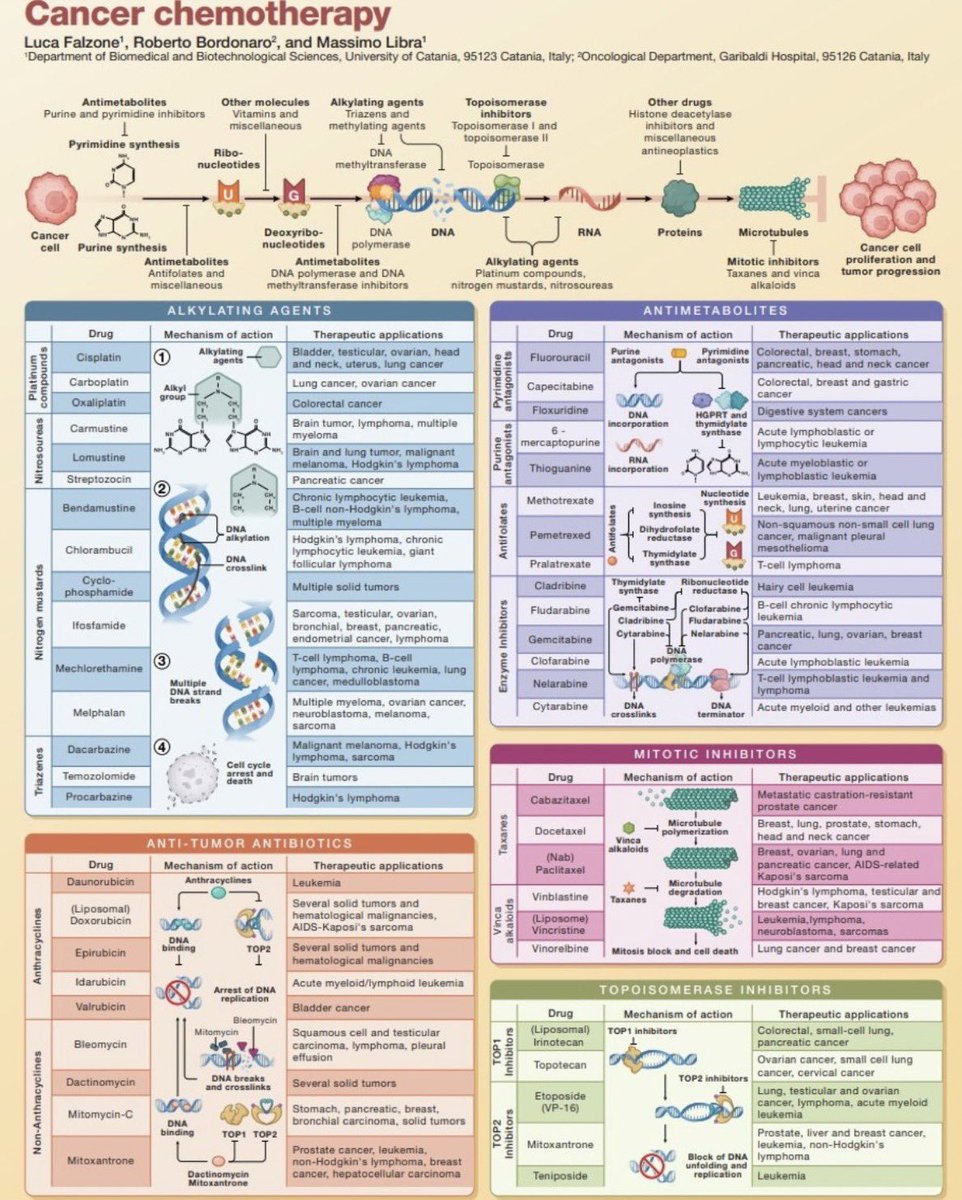
Today we report in Nature Genetics the genetic landscape of cancer drug resistance mechanisms from CRISPR base editing screens nature.com/articles/s4158…







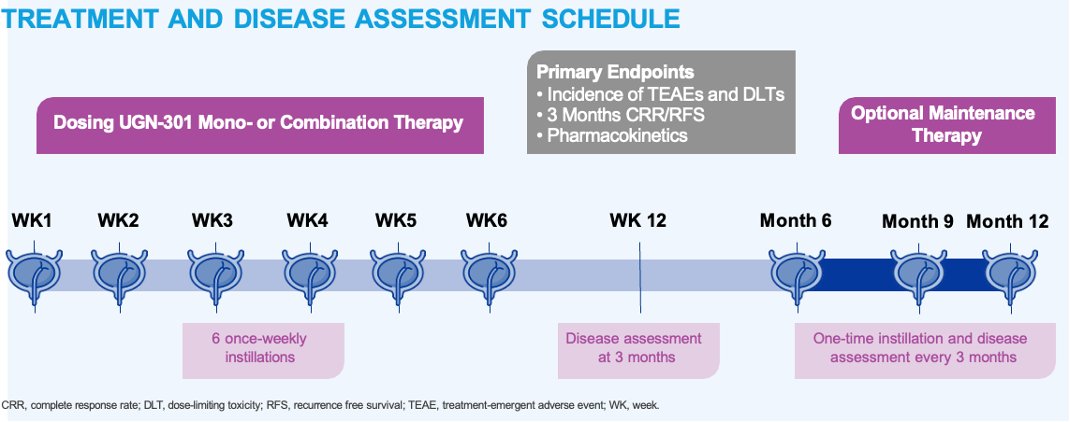
UR001: a phase 1 dose-escalation study of UGN-301 (Zalifrelimab) in patients with recurrent #NMIBC. Presentation by Marco Maruzzo IOV - Istituto Oncologico Veneto. #SUO24 written coverage by Zach Klaassen Georgia Cancer Center on UroToday > bit.ly/3B5MFSB Society of Urologic Oncology










TAR-200 monotherapy in patients with BCG–unresponsive high-risk #NMIBC carcinoma in situ: 1-year durability and patient-reported outcomes from SUNRISE-1 trial. Presented by Joseph Jacob, MD, MCR Upstate Cancer Center. #AUA25 written coverage by Julian Chavarriaga University of Toronto >


TAR-200 monotherapy in patients with BCG–unresponsive papillary disease–only high-risk #NMIBC: first results from cohort 4 of SunRise-1. Presented by Félix Guerrero-Ramos. #AUA25 written coverage by Julian Chavarriaga > bit.ly/3GHjCa0 Amer. Urol. Assn. J&J Medical Affairs Oncology