Mals Mariappan
@mariappanlab
ID: 1195511562720727041
https://www.mariappanlab.com/ 16-11-2019 01:19:14
103 Tweet
233 Followers
176 Following



Honored & excited to be selected for Best of Biophysical Journal symposium Biophysical Society #BPS2023 Shout out to Mals Mariappan & @MoitrayeeLab for their pivotal contributions Yale Cell Biology biophysics.org/2023meeting/pr…


Multiple job opportunites in the Yale West Campus Cores - mass spec & flow cytometry. Join an established team of staff scientists in support of research across campus. Please RT buff.ly/3lbi9i8 #research #hiring #yale @YWCimagingcore

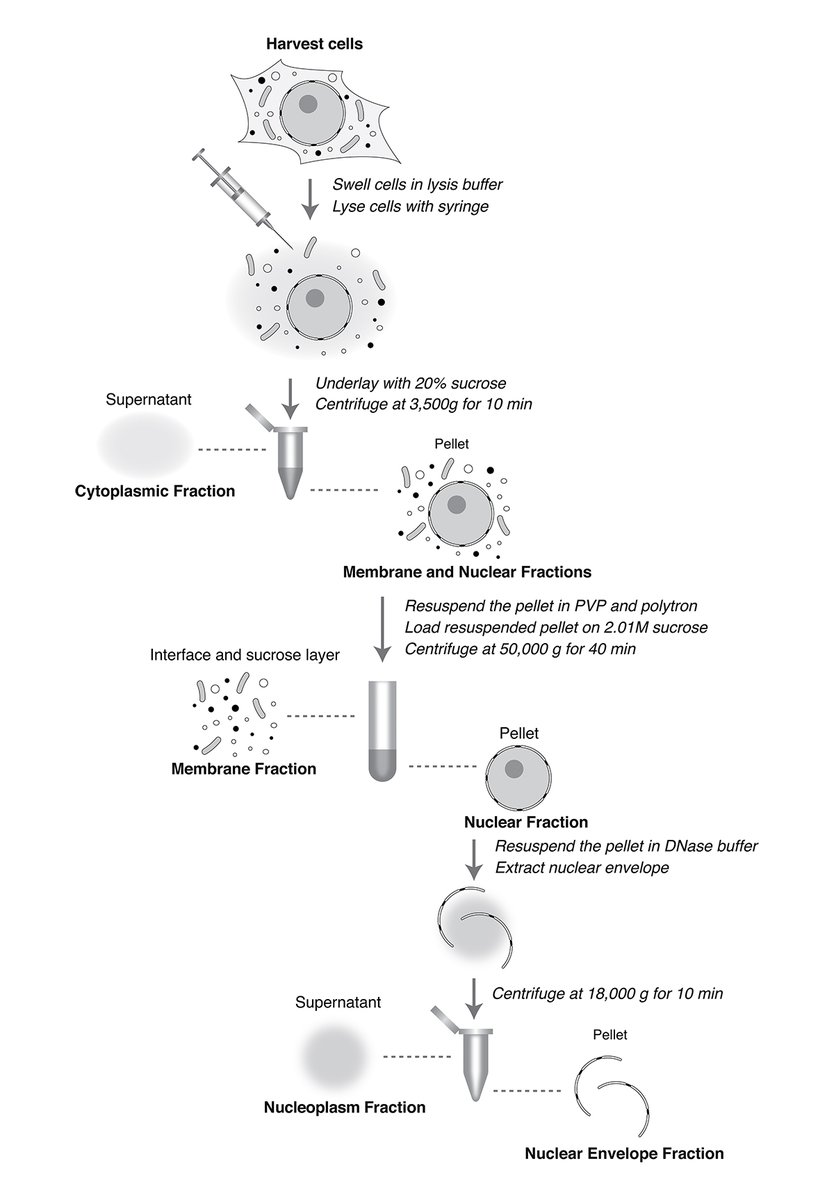
.yael udi et al. Rout Lab Rockefeller University present a new method for quantitative subcellular fractionation of a wide range of mammalian cells while maintaining nuclear integrity. bit.ly/404Jvpr Inna Ricardo-Lax #Biochemistry #Trafficking #Organelles

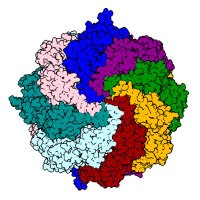
New work from Ron Kopito and Francesco Scavone reveal that UFMylation of ER translocon-bound 60S ribosome subunits promotes proteasome-mediated degradation of arrest polypeptides following ribosome stalling. 👏 biorxiv.org/content/10.110…

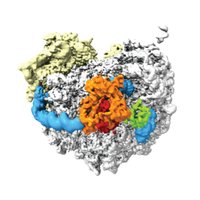

🚨Out now!🚨 Check out how cells adjust their tubulin mRNA levels in response to excess free tubulin. A 40-year-old puzzle solved! Wonderful collaboration with Eva Absmeier, Lori Passmore & Ivana Gasic 🧵 1/ cell.com/molecular-cell…



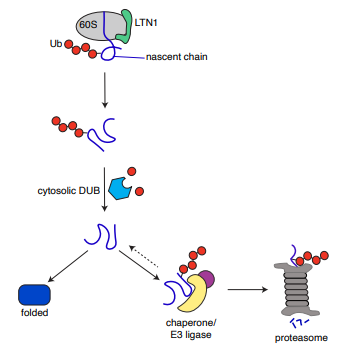
Nice reconstitution from Mals Mariappan. Nascent proteins from stalled ribosomes are polyubiquitinated and then promptly deubiquitinated, giving a second chance to fold. If a protein still can't fold, it exposes a degron, is re-ubiquitinated and degraded. biorxiv.org/content/10.110…