
Immune-Onc Therapeutics
@immuneonc
Science at Our Core. Patients in Our Hearts.
ID: 768245095619448834
https://www.immune-onc.com 24-08-2016 00:34:39
172 Tweet
243 Followers
55 Following

Today, we will be at the annual Stanford Drug Discovery Symposium. Yesterday, our CEO Charlene Liao had the pleasure of speaking with Dr. Brian Druker, recipient of the 2023 Stanford Drug Discovery Symposium Lifetime Achievement Award. If you're at #SDDS2023, please say Hello!


#DYK that myeloid checkpoints could be game-changing targets for the field of #cancerimmunotherapy? Join our CEO Charlene Liao's presentation at the Cambridge Healthtech Institute's 2nd Annual “Emerging Targets for Oncology and Beyond” conference at #PEGS23 next week. See you there!



Why are we so excited about #immunotherapy? Because we’re playing a big role in its evolution. This week at #PEGS23 our CEO, Charlene Liao discussed our scientific approach and robust pipeline targeting myeloid checkpoints. Learn more at Immune-Onc.com


Next Tue., May 23, our CEO Charlene Liao and CFO Chris Whitmore look forward to meeting with investors 1:1 at the 2023 Wells Fargo Virtual Private Biotech Symposium to discuss how our platform of #myeloid #checkpointinhibitors is reigniting the field of #immunotherapy. #biotech







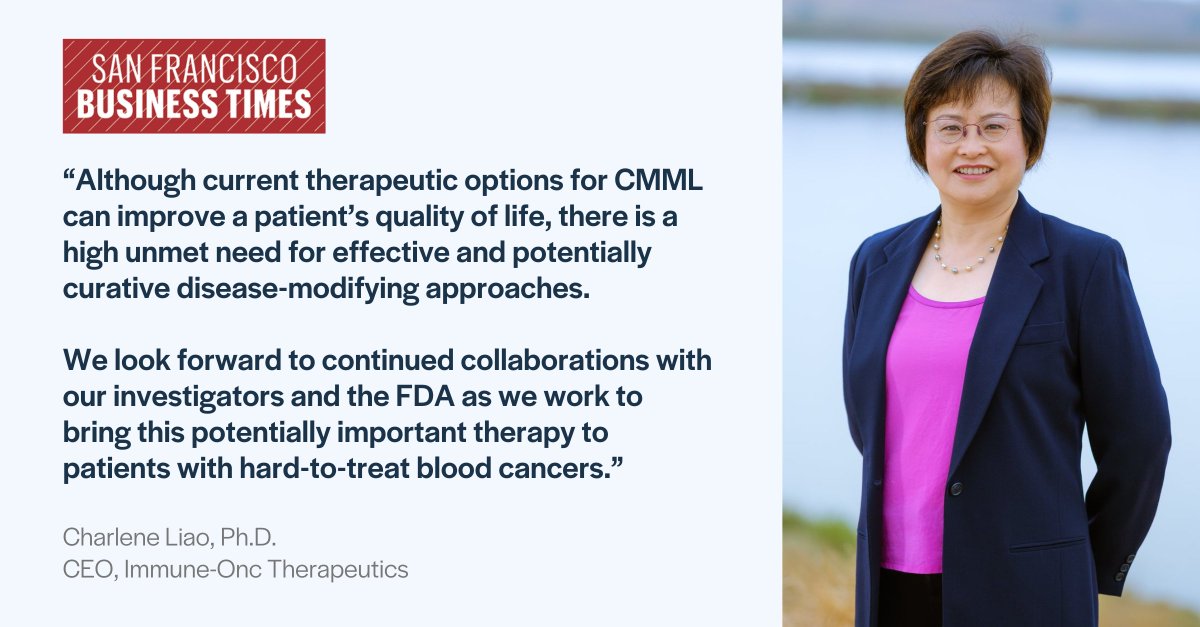
Exciting News! The San Francisco Business Times recognized our Orphan Drug Designation for IO-202 for #CMML as a recent breakthrough by women-led life sciences companies in the Bay Area. Read more here: bizjournals.com/sanfrancisco/n…


New from Immune-Onc Therapeutics at #EHA2024! Today, we will present additional positive interim Ph 1b expansion data for IO-202 in #CMML patients. The study shows early and sustained complete remissions among patients, regardless of their prognosis or mutation status. bit.ly/3Rs1SC7



Our Ph 1 data on IO-108 was published in Journal for ImmunoTherapy of Cancer ! · Durable responses in advanced solid tumors · Promising monotherapy & combo results with pembrolizumab · Potential to overcome resistance to checkpoint inhibitors Read the full press release: bit.ly/4fu6e5N


We’re proud to see Immune-Onc Therapeutics's IO-102 Ph 1 #CMML study featured in The Leukemia & Lymphoma Society's announcement highlighting new data showing progress for hard-to-treat blood cancers. Looking forward to sharing more updates on the study at #ASH2024. bit.ly/3Oqvvly

Immune-Onc Therapeutics will present important updates on its Phase 1b data on IO-202 for CMML. Don’t miss the oral presentation at #ASH2024 on Monday!














