
BioNTech SE
@BioNTech_Group
Our vision is to harness the power of the immune system to translate science into survival $BNTX https://t.co/eQJYOy56xw
ID:1060984350995550208
https://biontech.com/ 09-11-2018 19:55:56
271 Tweets
83,4K Followers
166 Following

We presented a positive data update for our #CARTcell therapy candidate BNT211 in advanced solid tumors at ESMO - Eur. Oncology Congress 2023. Our goal is to help improve the outcomes for a broad range of hard-to-treat tumors. Read more about the data update here: investors.biontech.de/news-releases/…



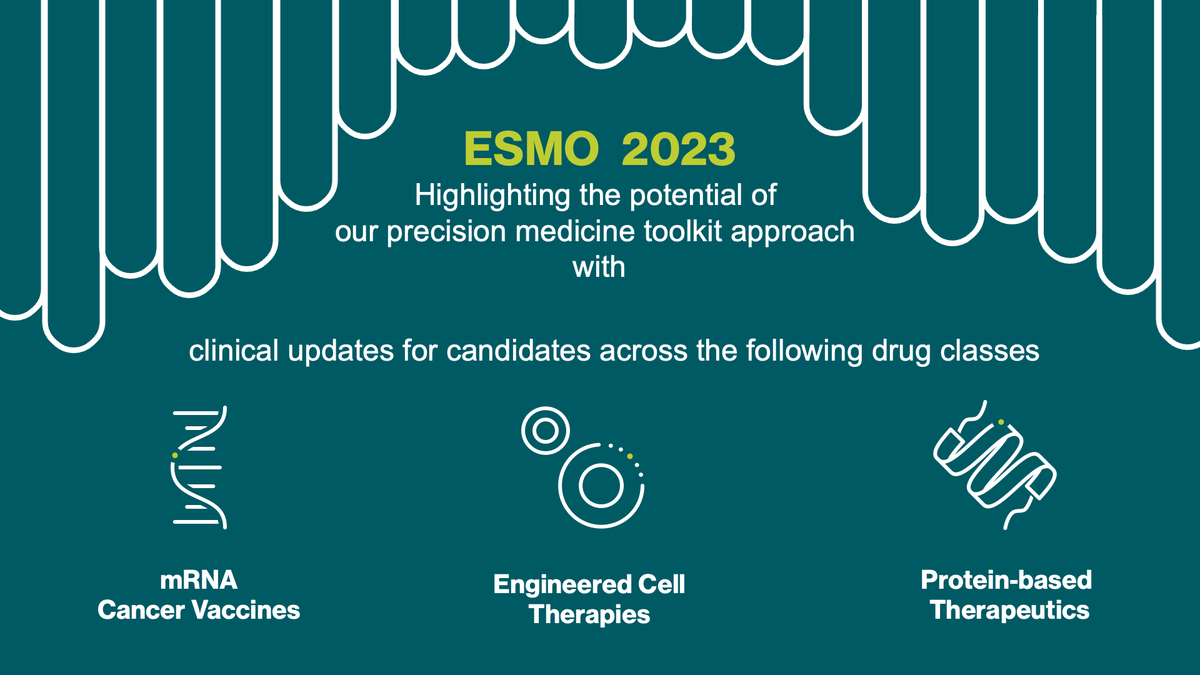
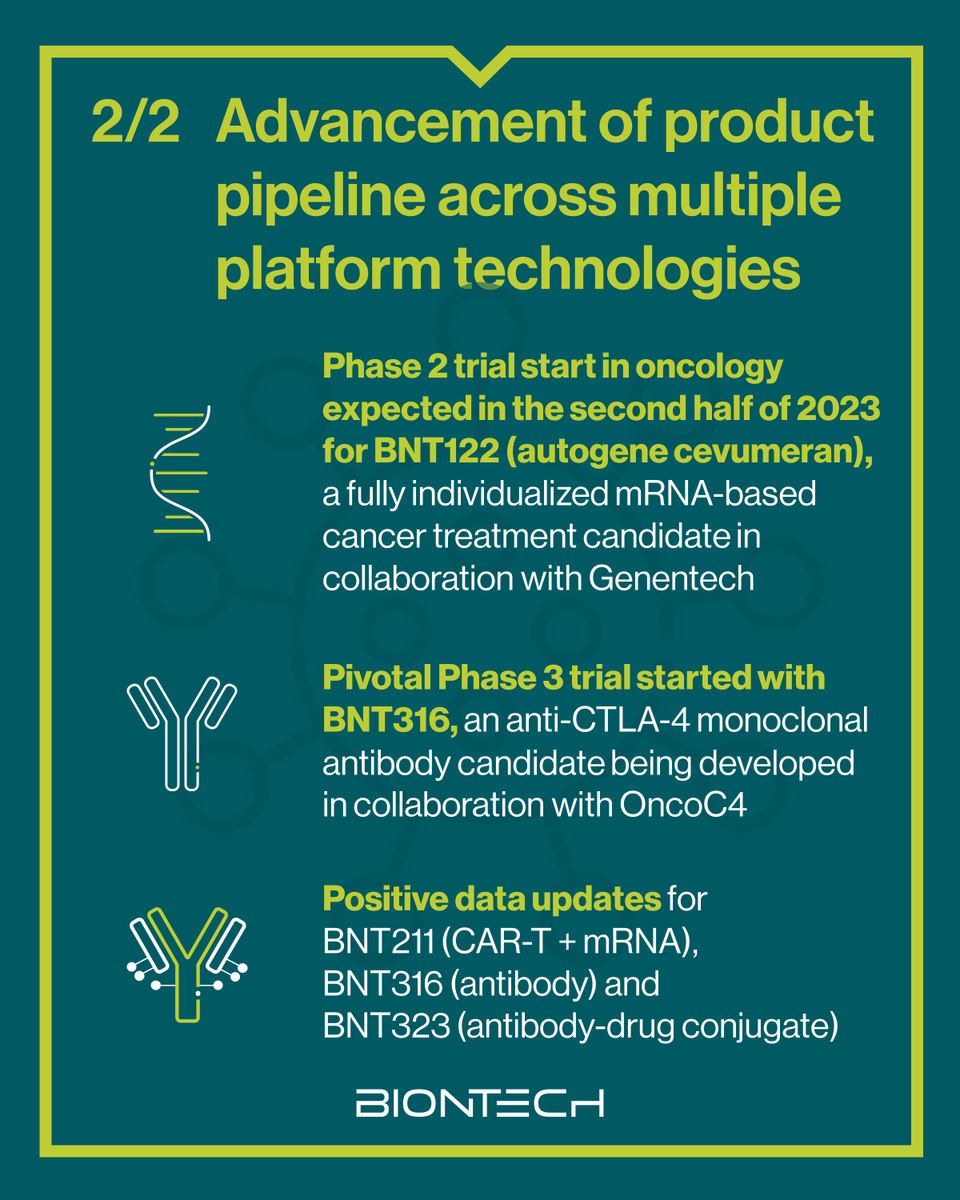
We are excited to present updates for five of our clinical candidates across our oncology pipeline at the 2023 ESMO - Eur. Oncology Congress. They underline the potential of our precision medicine toolkit for the treatment of solid tumors with high unmet medical need. investors.biontech.de/news-releases/…

We applaud Katalin Kariko and Drew Weissman on their #NobelPrize in Physiology or Medicine for pioneering nucleoside base modifications which were one of the key innovations applied to our #mRNA -based COVID-19 vaccine. Gratulálunk and congrats!

BioNTech has always been deeply rooted in academia. #STEM grads can now explore #science projects and apply for a full-time contract via our ATLAS #PhD program, a collab with TRON. Students with a sweet spot for #biomedical #research can apply by Oct 31. atlas-phdprogram.com/how-to-apply


The U.S. FDA just approved our Omicron XBB.1.5-adapted COVID-19 vaccine for individuals 12+ years & granted emergency use for individuals 6 month through 11 years. The updated vaccine will be available at pharmacies, hospitals & clinics across the US soon. investors.biontech.de/news-releases/…
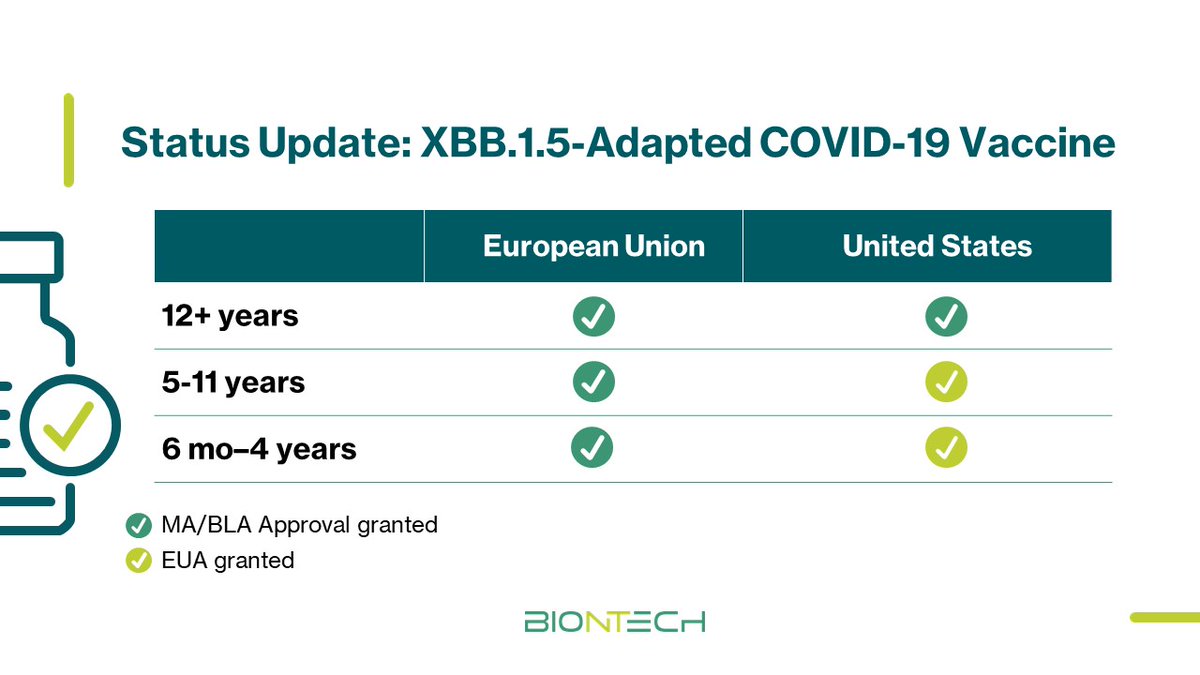

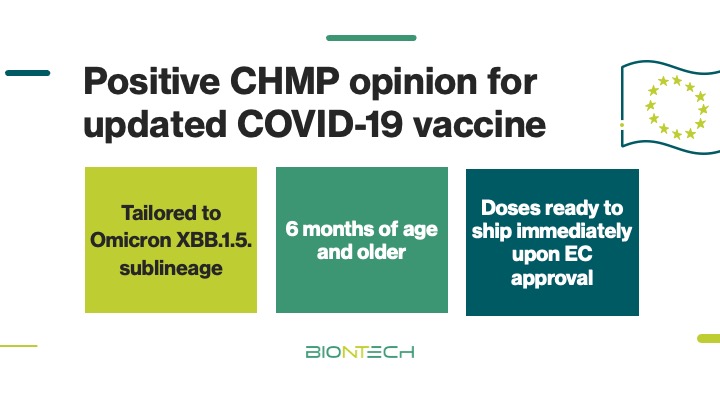
The EU Medicines Agency’ Committee for Medicinal Products for Human Use recommended marketing authorization of our XBB.1.5-adapted #COVID -19 vaccine. Following approval by the EC, the updated vaccine will be ready to ship to applicable EU member states immediately. investors.biontech.de/news-releases/…

#BioNTech expands its Management Board by appointing James Ryan as Chief Legal Officer. James will lead the legal strategy & global legal operations, contributing to BioNTech's success and growth trajectory in its mission to develop innovative #medicines . investors.biontech.de/news-releases/…


We enter H2/2023 with a strong financial position and have submitted applications for a variant adapted #COVID -19 vaccine to authorities. We aim to become a multi-product company by investing in our own clinical programs and adding compounds from partners. investors.biontech.de/news-releases/…

Welcome InstaDeep to BioNTech! The acquisition supports our strategy, aiming to build world-leading capabilities in #AI -driven drug discovery and development of next-gen #immunotherapies and vaccines to address diseases with high unmet medical need. investors.biontech.de/news-releases/…




