
BioDataScience Lab
@biodatasc
We apply our expertise in #Statistics and #ML to help answer principal questions in #microbiology and #microbiome.
Part of @LMU_Muenchen and @HelmholtzMunich.
ID: 1381604477849985027
https://bio-datascience.github.io/ 12-04-2021 13:46:13
45 Tweet
118 Followers
11 Following

Please, meet Roberto Olayo (Roberto), Sharma Lab, BrochadoLab and Victoria - a data generation 🤖 from the clip. They expect to have data for over 200k experiments for #StressRegNet project which is a part of bayresq.net ’s effort to tackle #antibiotic resistance💊💪.

We BioDataScience Lab have open positions for PhD/Postdocs at the interface of statistics, optimization, and microbiome data analysis #stats, #optimization, #microbiome, #datascience, #oceanmicrobiome #gutmicrobiome Computational Health Center Universität München; PM me or send an email for more info!!

Our lab Environmental Medicine Augsburg/Munich in Universität Klinikum #Augsburg. We SAW where skin #microbiome data comes from, and now will think twice before "df.dropna()" any samples🙀. Big thanks to @amedeodetomassi for an amazing lab tour, Luise Rauer and their colleagues for making it possible😎👍


That went by quickly! Already starting the last week of my three-month research stay at USC Marshall School of Business. Together with Jacob Bien and Christian L. Müller, we explored how to model compositional high-throughput sequencing data in a meaningful way. Stay tuned!


🔬 Today was an incredible day at Deng Lab! Huge thanks to Jinlong Ru, M. Khan Mirzaei, Roberto Olayo, Sophie Elizabeth Smith, and daniele pugno for sharing their expertise. Looking forward to new collaborations and more exciting discussions in the future🙌 #virome #microbiome Helmholtz Munich | @HelmholtzMunich


Discover the power of #NetCoMi, the #R package disentangling microbial association #networks! 📊🔬 🦠 Understand complex #microbial interactions 🌱 Statistical network estimation made easy 🔍 Unveil the differences between groups Don't miss a showcase made by Stefanie Peschel ⭐


🦠Unlocking Hierarchical Relationships in Viral #Metagenomic Data with #VirNest! 🧬 1️⃣ Virus gene-sharing network estimation by leveraging hierarchical protein association 2️⃣ Hierarchical partitions through nested stochastic block model Join daniele pugno in unveiling #microbiome


🔬🌿 Reproducible #microbiome #dataanalysis with #QIIME2 🐍 #python! 🔍 q2-gglasso: Sparse microbial network estimation 📈 q2-classo: Sparse log-contrast regression and classification Join Oleg Vlasovets MICROBIOME COSI to hear more about #openscience and #softwaredevelopment🚀💻



🔍Discover the power of PIMs (compositional power interaction models) in handling HiTSeq 2024 data! 🧬 @JohannesOstner showcases PIMs' capabilities in DA testing with correlated features while respecting zero inflation and compositional constraints 📈🎯 #ISMBECCB2023


🔬✨ Introducing MolE! Join Roberto Olayo to witness MolE's empowering chemical compound #analysis 🌐🌟 ⚙️ Molecular representation learned through embedding decorrelation ✨ Predicted growth-inhibitory effects against human gut pathogens #ML #antimicrobial #resistance #ISMBECCB2023


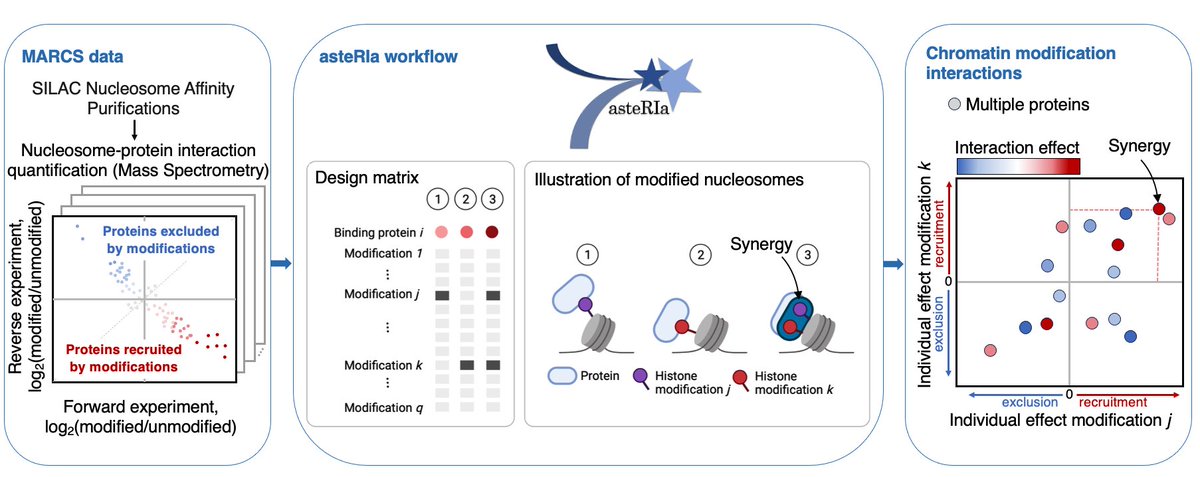
📊 Unveiling Stable Interactions in High-Throughput data! 💡Mara Stadler introduce #robust #statistical workflow: 🔗 Application to interaction effects between chromatin modifications 🧬 Lasso model for hierarchical interactions 🔎 Stability-based model selection #ISMBECCB2023




Excited to share our work "asteRIa enables robust interaction modeling between chromatin modifications and epigenetic readers", where we utilize the MARCS data for deeper insights into combinatorial epigenetics. Great collab between Bartke lab and BioDataScience Lab at Helmholtz Munich | @HelmholtzMunich

Considering microbial interactions in prediction tasks is crucial! 🦠📊 Excited about our collaboration with Christian L. Müller and Jacob Bien. Check out our pre-print: doi.org/10.1101/2024.0…


