
Catalent Pharma
@CatalentPharma
From drug and biologic development services to delivery technologies to supply solutions. Catalent. More products. Better treatments. Reliably supplied.™
ID:120578352
http://www.catalent.com 06-03-2010 22:04:29
10,7K Tweets
19,5K Followers
512 Following
Follow People





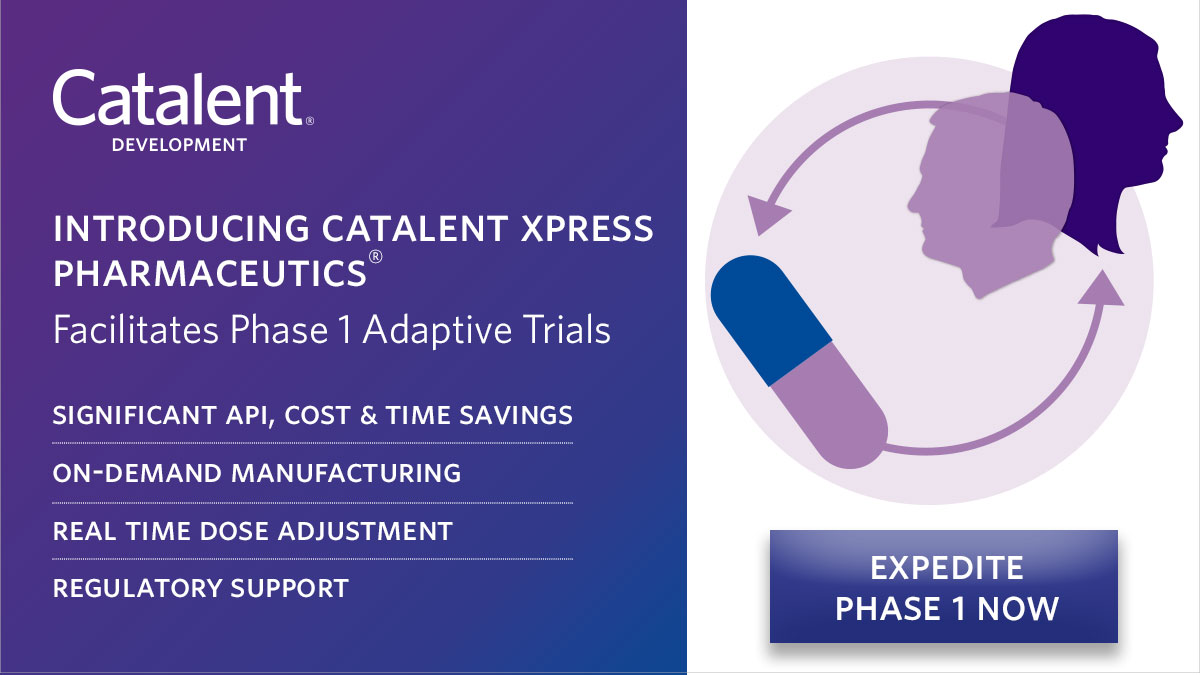
Introducing Catalent Xpress Pharmaceutics®: Formulation development with on-demand clinical manufacturing, regulatory support, and clinical testing for efficient First-in-Human studies. Fast-track to Phase 1 in 4-6 months! #AdaptiveTrials #DrugDevelopment ow.ly/fteH50RyKZU



Join us for ARM's 1.5-day workshop on Advanced Manufacturing in CGT, May 16-17, 2024, in Rockville, MD! Explore emerging technologies & innovations with Junxia Wang of Catalent. Don't miss it. Register now: ow.ly/Vgqc50RjrCo
#ARMWorkshop #CGT #AdvancedManufacturing


Schedule a meeting with our experts. Learn all about our integrated solutions for cell & gene therapies from raw material supply to clinic and commercial-scale manufacturing, plasmids, viral vectors, iPSCs, & autologous & allogeneic therapies. #ASGCT2024 ow.ly/UUCp50RxeL1



Streamline your vector development journey with Catalent's advanced tools and a pre-qualified analytical suite, ensuring high-yield, high-quality vectors without compromising on speed. Add Carey Connelly’s symposium to your schedule at #ASGCT2024 ! ow.ly/K9Ly50RwkeX


Learn why consumer health brands rely on Catalent to scale-up quickly and bring new products to market. Industry leading and experienced #Manufacturing , reliability supplied, something to chew on. #NutritionalSupplements ow.ly/X1gN50RwhVw






JunXia Wang will present at #ASGCT2024 on Catalent's new UpTempo℠ CAR-T Platform Process. This fully closed and GMP-compliant platform for the manufacturing of CAR-T cell therapies is a modular, plug-and-play model. Learn more here: ow.ly/hoSb50RuGQw









